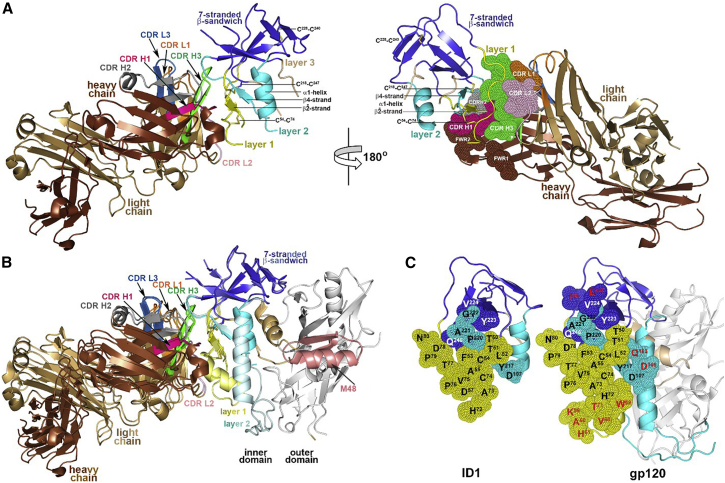
Figure 2.
Crystal Structure of the JR4 Fab-ID1 Complex
(A) The JR4 Fab-ID1 complex and its 180° view about a vertical axis are shown in a ribbon diagram with the molecular surface displayed over the residues of the Fab involved in ID1 binding (right). The structural gp120 elements engaged in JR4 Fab binding—the -,β4 strand and the α1 helix—are labeled.
(B) Structural comparison of JR4 Fab-ID1 and JR4 Fab-gp12093TH057 coree-M48 complexes. Complexes were aligned based on the ID of gp120 and are colored in darker and lighter shades for JR4 Fab-ID1 and the JR4 Fab-gp120 interface, respectively.
(C) JR4 epitope footprints on ID1 and the gp120coree. The gp120 residues buried at the complex interface are shown as spheres and displayed over the ribbon diagram of ID1 and the gp120coree. The gp120 residues that contribute to JR4 Fab binding in JR4 Fab-gp12093TH057 coree-M48 complex but are not involved in JR4 Fab binding to ID1 are shown in red.