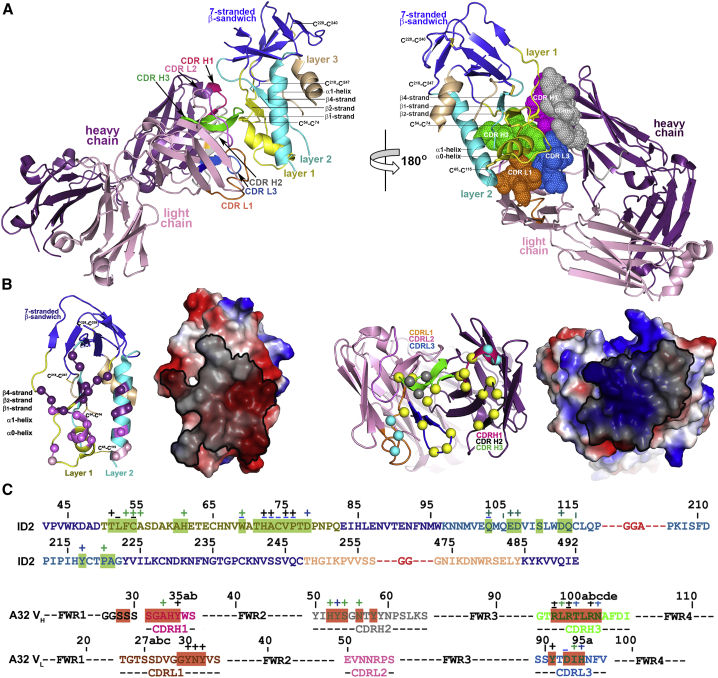
Figure 4.
Crystal Structure of A32 Fab-ID2 Complex
(A) A32-ID2 complex (left) and its 180° view about a vertical axis are shown in ribbon diagram with molecular surface displayed over the residues of the Fab involved in ID2 binding (right). See also Figure S3 and Table S1.
(B) Details of the A32 Fab-ID2 interface. A32 Fab contacts on ID2 and ID2 contacts on the A32 Fab are shown as balls displayed over the ID2/A32 Fab ribbon diagram and highlighted in black over the ID2/A32 Fab electrostatic potential surface. The A32 Fab contacts are shown in dark purple, light purple, and purple for heavy, light, and both chain(s) contacts, respectively. The ID2 contacts through residues of layer 1, layer 2, and both are shown in yellow, cyan, and gray, respectively.
(C) A32 Fab and ID2 contact residues on the primary sequence of ID2 and A32 Fab, respectively. Residues contributing to the A32 Fab-ID2 interface as defined by PISA (Krissinel and Henrick, 2007) are highlighted, and contacts as defined by a 5-Å cutoff are marked above the sequence. Side-chain (+) and main-chain (−) contacts are colored based on contact type; hydrophobic in green, hydrophilic in blue, or both in black.