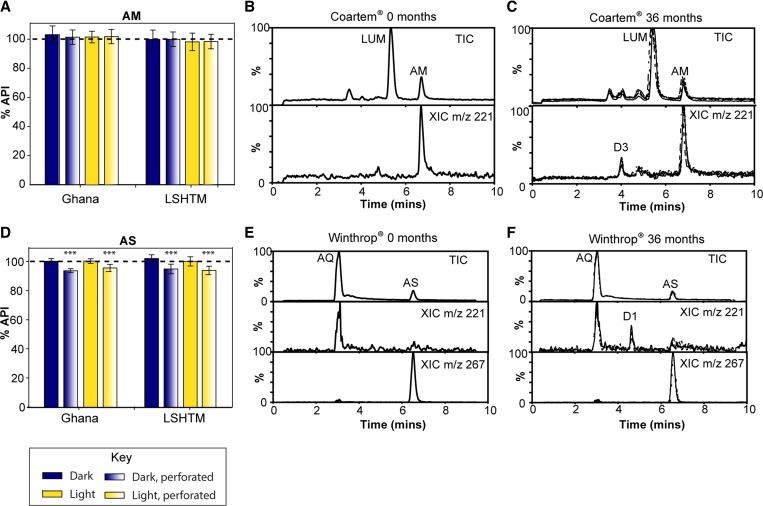
Figure 4.
Long-term stability of artemisinin-based combination therapies in tropical climates. AM content in AM/LUM tablets (A) and AS content in AS/AQ tablets (D) was measured at 36 months after storing at high temperature and humidity in Ghana or a pharmaceutical stability chamber (LSHTM). Error bars represent standard deviation (N = 36). AS/AQ tablets in perforated blister packs showed significantly lower levels of AS than those in which the blister packaging was intact (*** P < 0.001). Tablets were analyzed by liquid chromatography mass spectrometry and representative chromatograms are shown at the start (B, E) and end (C, F) of the study. Extracted chromatograms (m/z 221) show the presence of degradation product D3 in aged AM/LUM and D1 in aged AS/AQ tablets. AM/LUM = artemether/lumefantrine; AS/AQ = artesunate/amodiaquine; DHA/PIP = dihydroartemisinin/piperaquine; LSHTM = London School of Hygiene and Tropical Medicine.