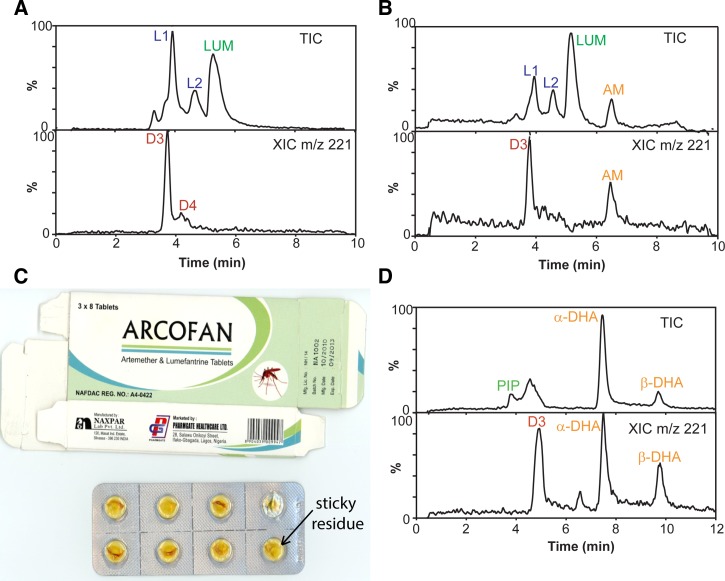
Figure 5.
Comparing degraded tablets (field samples) with artificially degraded tablets. Liquid chromatography mass spectrometry (LC–MS) analysis of AM/LUM Arcofan (A) and Amatem Tab® (B) tablets revealed similar degradation products to artificially degraded AM/LUM tablets (“forced degradation”). Extracted ion chromatograms for m/z 221 (signature fragment ion) are shown below the total ion chromatogram. Examination of the Arcofan packaging revealed a sticky residue on the inside of the blister packs (C). LC–MS analysis of Droa-Quine® DHA/PIP tablet revealed the presence of degradation product D3 (D). AM/LUM = artemether/lumefantrine; DHA/PIP = dihydroartemisinin/piperaquine.