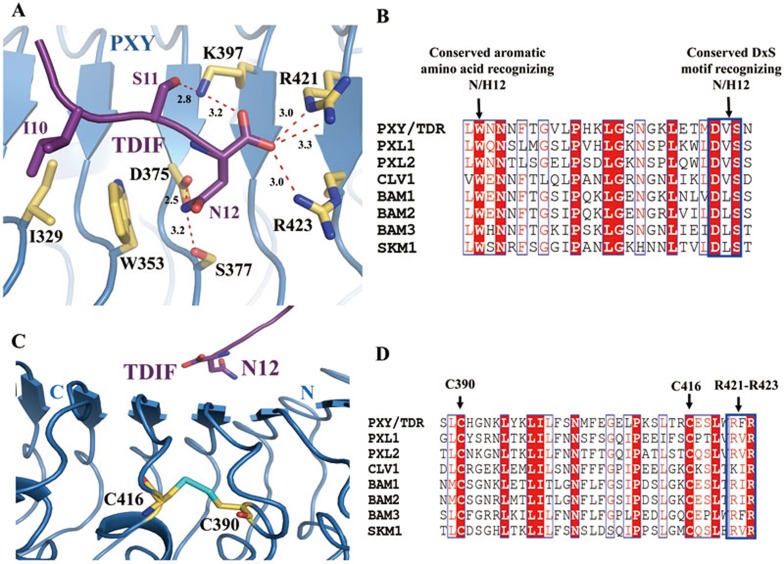
Figure 4.
The last residue of TDIF is recognized by a set of highly conserved amino acids of PXY. (A) Detailed interactions of the conserved TDIFAsn12 with PXYLRR. The side chains of some amino acids from TDIF and PXYLRR are shown in purple and yellow orange, respectively. Red dashed lines indicate hydrogen bonds or salt bridges. (B) Sequence alignment of PXYLRR with other CLE receptors around the TDIFAsn12-interacting region. (C) The two consecutive LRRs recognizing the last residue of TDIF are stabilized by a conserved disulfide bond. The sulfur atoms are colored in cyan. (D) Sequence alignment of PXYLRR with other known CLE receptors around the disulfide bond region. For (B and D), conserved and similar residues are boxed with red ground and red font, respectively. Conserved motifs are boxed with blue frames.