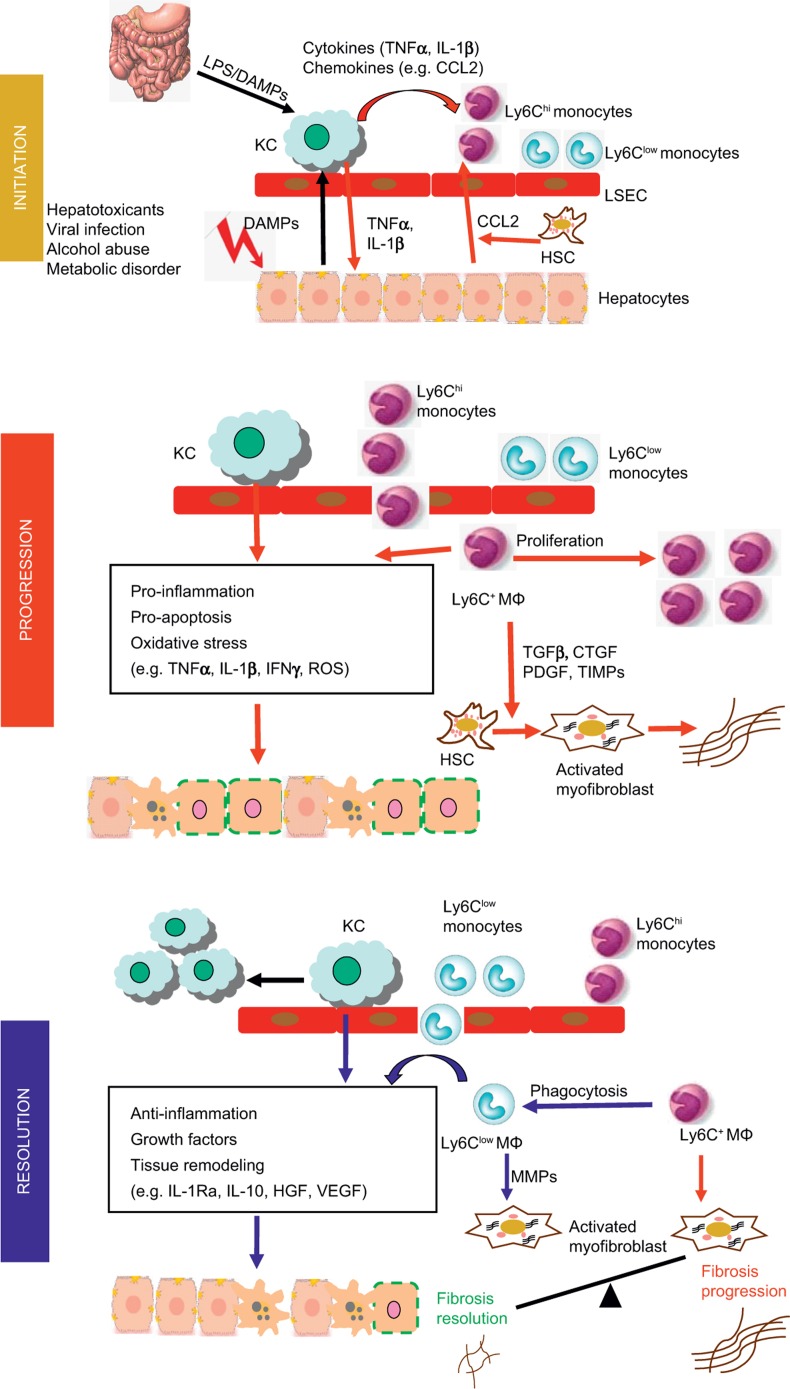
Figure 1.
Involvement of hepatic macrophage populations during the initiation, progression and resolution of liver injury in mouse models. In the initial phase, hepatocyte damage triggers the release of damage-associated molecular pattern molecules (DAMPs) which stimulate Kupffer cell (KC) activation activation. Endotoxin from the gut–liver axis may additionally endorse Kupffer cell activation. Activated Kupffer cells secrete pro-inflammatory cytokines, including IL-1β and TNFα, which contribute to hepatocyte injury. Hepatocytes, stellate cells and Kupffer cells secrete chemokines such as CCL2 that promotes the recruitment of Ly-6Chi monocytes into the liver, where they develop into Ly-6C+ macrophages. These cells promote the progression of liver injury by secreting pro-inflammatory cytokines and producing ROS. During chronic injury, Ly-6C+ macrophages also trigger HSC activation and promote myofibroblast production of extracellular matrix through releasing pro-fibrotic mediators, such as tumor growth factor (TGF)-β, connective tissue growth factor (CTGF), PDGF, and tissue inhibitor of matrix metalloproteinase (TIMPs). Phagocytosis of dead cells initiates the resolution of inflammation and tissue restoration. Upon phagocytosis or other resolution stimuli, the Ly-6Chi macrophages switch to Ly-6Clow macrophages that exhibit a restorative phenotype. The Ly-6Clow macrophages, as well as Kupffer cells produce anti-inflammatory mediators, such as IL-1 receptor antagonist (IL-1Ra) and IL-10. They release growth factors, including hepatocyte growth factor (HGF) and VEGF. Moreover, the Ly-6Clow macrophages produce matrix metalloproteinases (MMP-9, -12, -13) and promote the degradation of excessive extracellular matrix proteins. Overall, the phenotype and functional heterogeneity of hepatic macrophages play critical roles in determining the balance between the mechanisms of progression and resolution of tissue injury during both acute and chronic liver injuries.