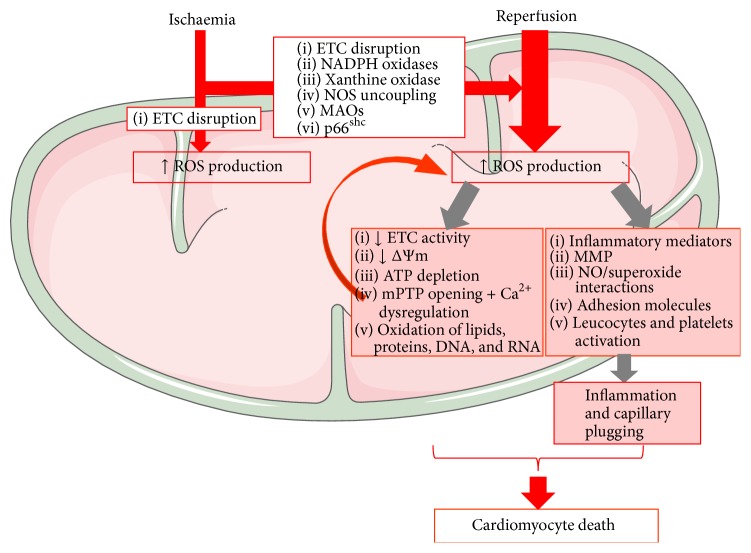
Figure 1.
Mitochondrial ROS contribution to I/R injury. Cellular hypoxia secondary to ischaemia results in disruption of ETC activity in the IMM (inner mitochondrial membrane) with subsequent ROS production. Increased activity of MAOs, NADPH oxidase, and p66shc; conformational changes of xanthine oxidase; and/or NO synthase uncoupling further amplify ROS production upon reoxygenation. Increased mitochondrial ROS damages mtDNA and RNA with ETC impairment. Dysfunctional ETC will amplify ROS generation, leading to a vicious cycle of mitochondrial cumulative damage, decreased mitochondrial membrane potential (Δψ m) and respiration, mPTP opening with cellular swelling and Ca2+ dysregulation, and oxidation of lipids and proteins. Postischaemic ROS generation also stimulates an inflammatory response, with the release of chemical mediators and expression of adhesion molecules by endothelial cells and leukocytes. ROS-dependent activation of MMPs (matrix metalloproteinases) is also responsible for the functional impairment of several proteins and receptors. The inflammatory response and the activation of leucocytes and platelets trigger the narrowing of capillaries during reperfusion, accelerating the progression towards cardiomyocyte death. (Illustration realized thanks to Servier Medical Art.)