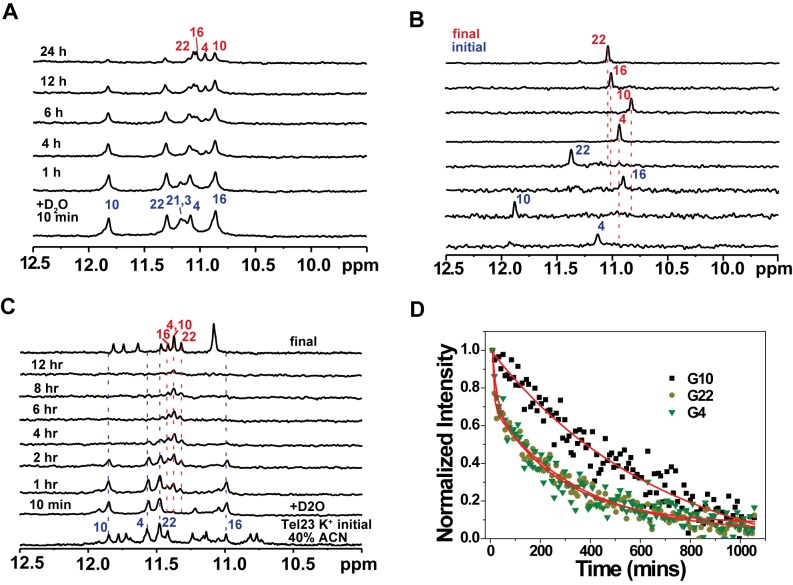
Figure 2.
NMR spectroscopy with HDX of BMVC-8C3O or acetonitrile induced conformational changes in a Tel23 G4. (A) NMR-HDX spectra of the Tel23 G4 with BMVC-8C3O. At the initial time point, Tel23 G4 sample in the presence of 150 mM K+ was lyophilized after addition of 5 eq. BMVC-8C3O and then dissolved in 99% D2O immediately before NMR measurement, followed by recording of spectra at 25°C at 10 min; at 1, 4, 6, 12 and 24 h, in the ascending order. (B) Site-specific assignment of imino proton resonances in the initial and final forms of the central quartet of Tel23 during interaction with the BMVC-8C3O. The 1D 15N-1H SOFAST-HMQC spectra of 16% 15N-enriched Tel23 G4 samples are shown with the assignment and site-specific labeling that correspond to the labeled sites. (C) Imino proton NMR spectra of the Tel23 G4 in the presence of 150 mM K+ after addition of 40% v/v acetonitrile 10 mins (bottom) and after annealing, and stacked plot with NMR-HDX spectra, in which lyophilized Tel23 G4 sample in the presence of 150 mM K+ dissolved in D2O and 40% v/v acetonitrile-d3, and immediately before NMR measurement, followed by recording of spectra at 25°C at 10 min; at 1, 4, 6, 12 and 24 h. (D) The NMR-HDX curves of G-4, G-10 and G-22; these data were extracted from the imino proton spectra and analyzed by bi-exponential fitting.