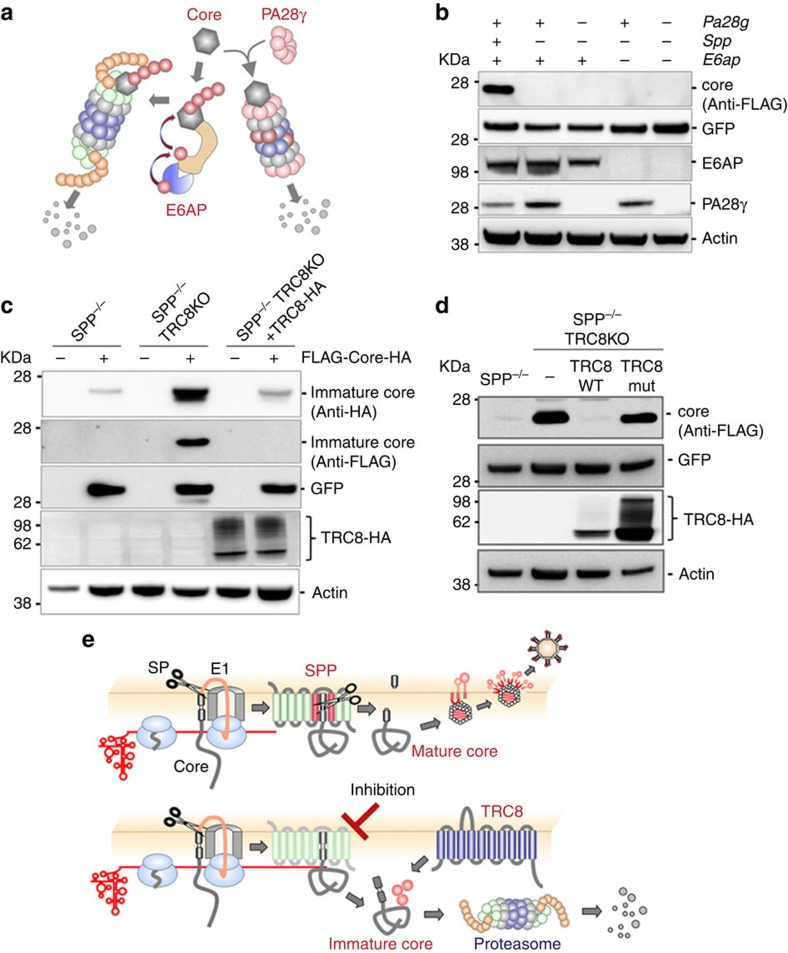
Figure 6. TRC8-dependent proteasome degradation of the immature core protein in SPP-deficient cells.
(a) Schematic representations of two distinct pathways of HCV core protein degradation. The mature core protein interacts with PA28γ in the nucleus and is degraded by the proteasome independent of ubiquitin52. In contrast, E6AP can also bind to the mature core protein in the perinuclear region. E6AP ubiquitinates the core protein and degrades it in an ubiquitin-dependent manner34. (b) SPP/PA28γ/E6AP triple-knockout MEFs (SPP/PA28γ/E6APTKO) expressing FLAG-HCV core via lentivirus were subjected to immunoblotting at 48 h post infection. (c) SPP−/− MEFs, SPP−/−TRC8KO MEFs and SPP−/−TRC8KO MEFs restored by HA-tagged TRC8 expression were infected with a lentivirus expressing FLAG-core-HA and subjected to immunoblotting. (d) SPP−/− and SPP−/−TRC8KO MEFs complemented with TRC8-HA or TRC8mut-HA and expressing FLAG-core were subjected to immunoblotting. (e) Maturation of the HCV core protein by processing with SP and SPP (upper panel). SPP deficiency and treatment with an inhibitor for SPP induced the degradation of the immature core protein by the proteasome, mediated by the E3 ligase TRC8 (lower scheme). The data are representative of two independent experiments.