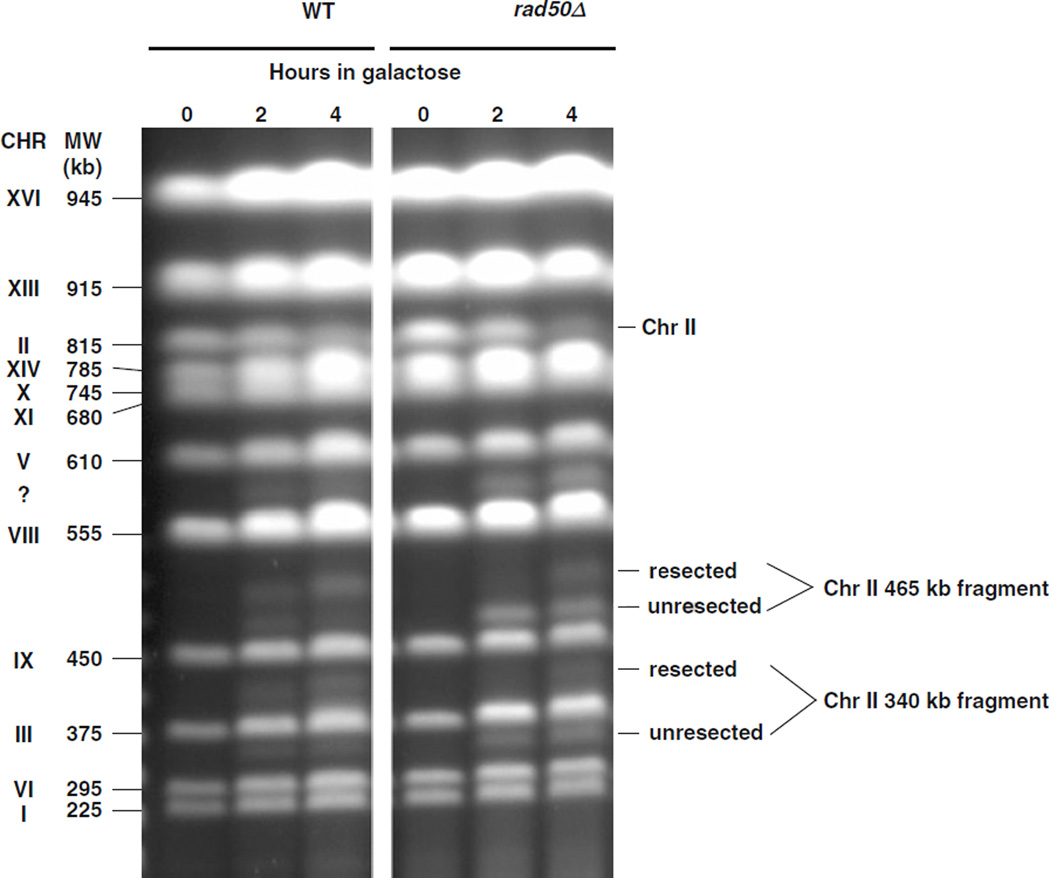
Fig. 2.3.
PFGE-shift of chromosome fragments generated by an I-SceI site-specific break is detected on ethidium bromide stained gels. The galactose-inducible I-SceI endonuclease that cuts at a specific site engineered into Chr II was induced by transferring cells to galactose (see (23)). Samples were taken at 0, 2, and 4 h and analyzed by PFGE. The I-SceI site is on Chr II (815 kb) and cuts the DNA into 340 and 465 kb fragments. The efficiency of I-SceI cutting was 60% in WT and 80% in a rad50-null mutant at 4 h. Most of the fragments generated in WT cells within 4 h after transferring to galactose were shifted on PFGE (left image). However, for the rad50 mutant, less than half of the molecules were shifted (right half of image). These results are consistent with those described by Westmoreland et al. (19) using an HO endonuclease acting at a different site and demonstrate with the PFGE-shift approach a role for the MRX complex in resection. (We note that in these experiments an unidentified fragment appeared between 555 and 610 kb as shown by the symbol “?” The origin of this cryptic target remains to be determined but the site of cutting is likely highly related to the I-SceI site.) Experimental protocol: The experiment was performed at 30°C. Cells were grown overnight in YPDA medium, resuspended in YEP lactate medium (3.15% lactic acid, pH 5.5), and grown for an additional 18 h. The cells were then transferred to synthetic lactate medium (3.15% lactic acid, pH 5.5) containing 2% galactose. Cells were harvested at 0, 2, and 4 h and plugs were prepared for PFGE as described in the text.