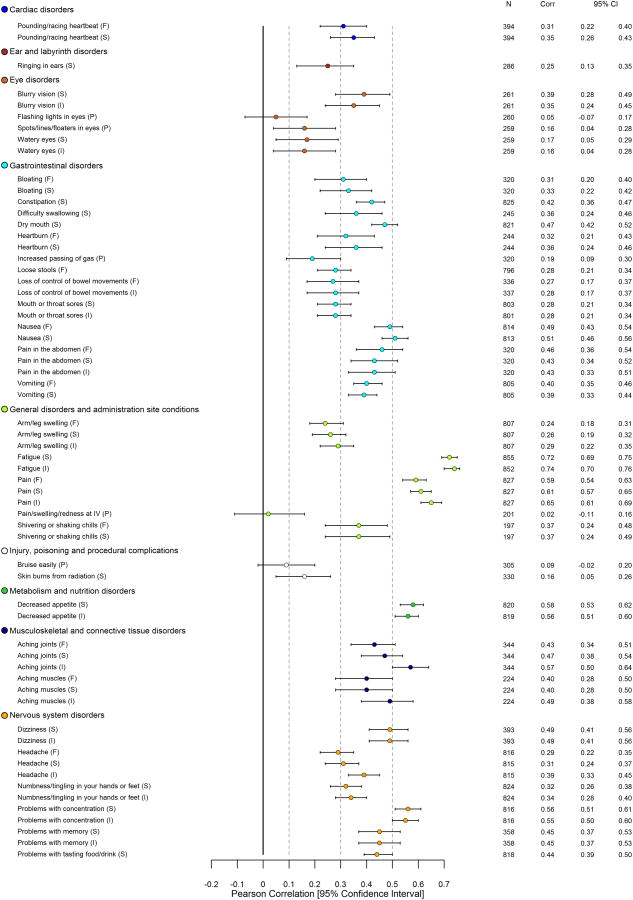
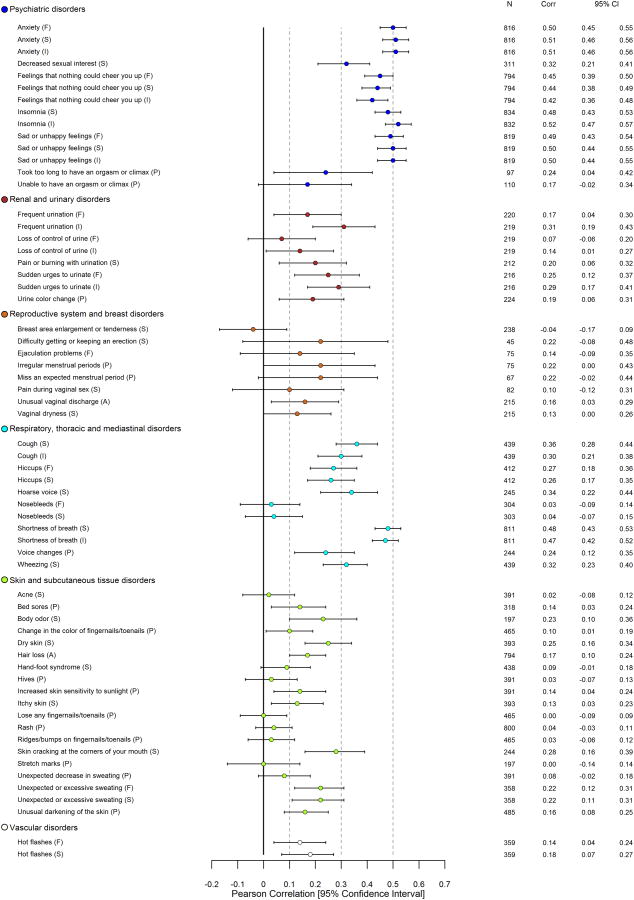
Figure 1. Pearson Correlations between 124 PRO-CTCAE Item Scores and EORTC QLQ-C30 HRQOL Summary Score* at Visit 1.
Abbreviations: PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire; HRQOL, Health-related quality of life; CTCAE, Common Terminology Criteria for Adverse Events
*See eTable 3 for all computed Pearson correlations between PRO-CTCAE items and EORTC QLQ-C30 functioning, global, and symptom scales.