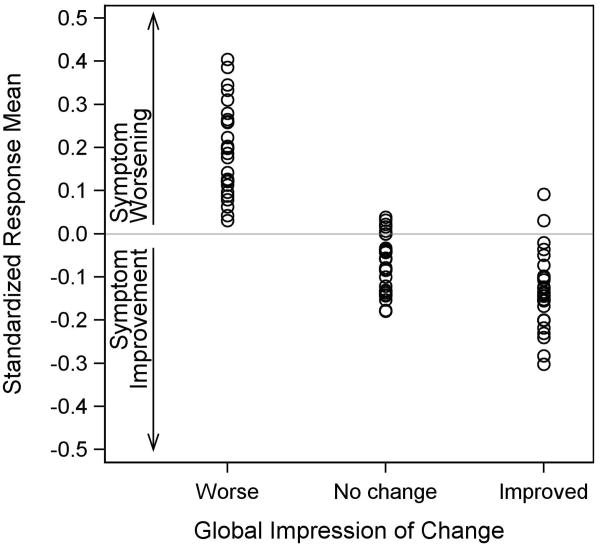
Figure 2. Standardized Response Means across 27 PRO-CTCAE Items by Patient-Reported Global Impression of Change Category*.
Abbreviation: PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events
*Figure 2 includes 27 frequency, severity, and interference items selected prior to initiation of the responsiveness analysis. The set of 20 “core” symptomatic AEs was reviewed and symptomatic AEs were selected if they had high potential to be meaningfully related to global changes in quality of life, physical condition, and/or emotional state (i.e., the Global Impression of Change items which were administered at the second visit). Of the 20 reviewed symptomatic AEs, 13 were included based on this criterion (see eTable 2). The symptomatic AEs which were excluded were felt to be related to initiation or changes in specific treatments (dry mouth, problems with tasting food/drink, rash) so may not exhibit change in a heterogeneously treated sample of patients; require a longer duration of follow-up to exhibit change (arm/leg swelling, hair loss); or be related to cognitive condition (headache, problems with concentration) which was not assessed in the Global Impression of Change items.