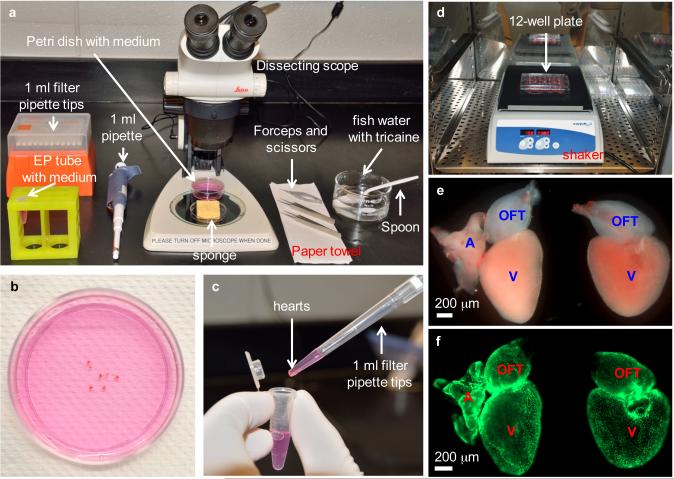
Figure 2.
Heart dissection and explant culture. (a) Equipment setup for heart dissection, including dissecting scope, forceps, scissors, pipette and filter tips, grooved sponge, 60 mm Petri dish with culture medium, 1.5 ml EP tube with culture medium, finger bowel with diluted Tricaine in aquarium water, spoon and paper towel. (b) Collected hearts are placed in a 60 mm Petri dish to remove blood clots and extra tissues. (c) Cleaned hearts are transferred to an EP tube by using a 1 ml pipette tip with the end cut off. (d) Washed heart explants are transferred to a 12-well culture plate, and the plate is placed on a shaker in a CO2 incubator, shaking at 150 rpm. (e) Close-up view of two heart explants with (left) or without (right) outflow tract in a culture plate. Hearts are from tcf21:nucEGFP zebrafish. A, atrium; V, ventricle; OFT, outflow tract. (f) Fluorescent view of explants in (e), showing nuclear EGFP signals. Work with zebrafish was approved by the Institutional Animal Care & Use Committee at Duke.