Abstract
Aims
In the presence of oxygen, most of the synthesized pyruvate during glycolysis in the cancer cell of solid tumors is released away from the mitochondria to form lactate (Warburg Effect). To maintain cell homeostasis, lactate is transported across the cell membrane by monocarboxylate transporters (MCTs). The major aim of the current investigation is to identify novel compounds that inhibit lactate efflux that may lead to identifying effective targets for cancer treatment.
Study Design
In this study, 900 ethanol plant extracts were screened for their lactate efflux inhibition using neuroblastoma (N2-A) cell line. Additionally, we investigated the mechanism of inhibition for the most potent plant extract regarding monocarboxylate transporters expression, and consequences effects on viability, growth, and apoptosis.
Methodology
The potency of lactate efflux inhibition of ethanol plant extracts was evaluated in N2-A cells by measuring extracellular lactate levels. Caspase 3- activity and acridine orange/ethidium bromide staining were performed to assess the apoptotic effect. The antiproliferative effect was measured using WST assay. Western blotting was performed to quantify protein expression of MCTs and their chaperone CD147 in treated cells lysates.
Results
Terminalia chebula plant extract was the most potent lactate efflux inhibitor in N2-A cells among the 900 - tested plant extracts. The results obtained show that extract of Terminalia chebula fruits (TCE) significantly (P = 0.05) reduced the expression of the MCT1, MCT3, MCT4 and the chaperone CD147. The plant extract was more potent (IC50 of 3.59 ± 0.26 μg/ml) than the MCT standard inhibitor phloretin (IC50 76.54 ± 3.19 μg/ml). The extract also showed more potency and selective cytotoxicity in cancer cells than DI-TNC1 primary cell line (IC50 7.37 ± 0.28 vs. 17.35 ± 0.19 μg/ml). Moreover, TCE Inhibited N2-A cell growth (IG50 = 5.20 ± 0.30 μg/ml) and induced apoptosis at the 7.5 μg/ml concentration.
Conclusion
Out of the 900 plant extracts screened, Terminalia chebula ethanol extract was found to be the most potent lactate efflux inhibitor with the ability to inhibit chaperone CD147 expression and impact the function of monocarboxylate transporters. Furthermore, TCE was found to have growth inhibition and apoptotic effects. The results obtained indicate that Terminalia chebula constituent(s) may contain promising compounds that can be useful in the management of neuroblastoma cancer.
Keywords: Plant ethanol extracts, monocarboxylate transporters, CD 147, lactate inhibitor, apoptosis, growth inhibition
1. INTRODUCTION
Unlike normal cells, solid tumor relies on aerobic glycolysis as the primary source of energy, a phenomenon known as the Warburg Effect [1]. As the end-product of glycolysis, lactate is produced in an excessive amount [2] and considered an alternative source of fuel for the uncontrolled cell proliferation [3]. Lactate efflux to the cell microenvironment is critical to cell survival. The extracellular acidosis of the cancer cell was found to enhance cell invasiveness [4], metastasis [5], and chemotherapy resistance [6]. On the other hand, the continuous lactate production will cause intracellular acidosis. The acidic intracellular pH will eventually initiate apoptosis [7, 8] through different mechanisms such as promoting the permeability of mitochondria membrane [9], activating endonucleases that cause DNA fragmentation [10], or activating caspase-3 protease, the key indicator of apoptosis that deactivates essential metabolic proteins [11].
The mammalian cell has many transporters involved in the regulation of pH homeostasis [12]. However, monocarboxylate transporters (MCTs) are considered the most important pH cell regulators, especially within tumor cells with rapid metabolism and high glycolysis rate [13]. These MCTs (also known as solute carrier 16, SLC16 proteins) are a family of 14 transporters, and the first four members (MCT1-MCT4) documented as single-carboxylate molecules transporters across the biological membranes [14]. MCT1 is considered high-affinity lactate transporter involved in exogenous lactate uptake by the cancer cells [15] that facilitate lactate efflux according to pH gradient [16]. On the other hand, the low-affinity lactate transporters MCT4 release lactate [2]. Moreover, it was recently reported that MCT3 is involved in lactate efflux of some cells [17].
On the other hand, natural products have played a very important role as cancer chemotherapeutic agents [18]. Specifically, natural flavonoids were found as MCTs inhibitors [19]. MCTs are attractive targets for cancer therapy, especially in cancers of a hyper-glycolytic and acid-resistant phenotype [20]. Therefore, this study was designed to identify potent natural lactate efflux inhibitors among 900 plant extracts and to explore their mode of inhibition. Furthermore, the consequential effects of these extracts on cell viability, proliferation, and apoptosis were also examined.
2. METHODOLOGY
Screened plants and herbs were obtained from our “FAMU Herbal Resource Facility” where we have over 1100 stored medicinal plants. The facility is located within our research laboratory. The plants were originally obtained from several sources including Frontier Natural Products Coop (Norway, IA, USA), Monterey Bay Spice Company (Watsonville, CA, USA), Mountain Rose, Herbs (Eugene, OR, USA), Mayway Traditional Chinese Herbs (Oakland, CA, USA), Kalyx Natural Marketplace (Camden, NY, USA), Futureceuticals (Momence, IL, USA), Organic Fruit Vegetable Markets and Florida Food Products Inc. (Eustis, FL, USA). L-lactate assay kits were obtained from Eton Bioscience (San Diego, CA, USA), and water-soluble tetrazolium (WST) proliferation assay kits from GBiosciences (St. Louis, MO, USA). EnzChek® Caspase-3 Assay were purchased from Life Technologies Inc., (Grand Island, NY, USA). Resazurin (7-hydroxy-10-oxido-phenoxazin-10-ium-3-one), a-cyano-4-hydroxycinammic acid (CHC), phloretin and absolute ethanol were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Other laboratory supplies were obtained from VWR International (Radnor, PA, USA), Atlanta Biological (Flowery Branch, GA, USA), and Santa Cruz Biotechnology, Inc. (Dallas, TX, U.S.A). Primary antibodies monocarboxylate transporter 1(MCT1), monocarboxylate transporter 3 (MCT3), monocarboxylate transporter 4 (MCT4), Basigin (CD147), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), secondary antibody and chemiluminescence reagent, were provided by Abcam (Cambridge, MA, USA). Pierce protein assay kit was purchased from Thermo Scientific (Rockford, IL, USA). Bio- Rad (Hercules, CA, USA) supplied running and transferring buffers, standard protein ladder, Laemmli sample buffer, and nitrocellulose. RIPA lysis buffer and mammalian protease arrest were obtained from G-Biosciences (St. Louis, MO, USA).
2.1 Plant Extraction
The screened plants were extracted with ethanol, the most common and safe organic solvents in pharmacological studies evaluating the activity of medicinal herbs [21]. Briefly, the selected plants were grounded, homogenized in 99.5% ethanol, and then placed in the dark on a shaker for 24 h at RT. Plant-ethanol mixture stored in air tight 15 ml glass containers at −20°C in the dark until the time of the study. Further, the identified plant extract for more investigation, Terminalia chebula fruits (TCE) was finely grounded and extensively extracted by soaking in 99.5% ethanol for seven consecutive days on a shaker in dark and at RT. The plant-ethanol mixtures were filtered and dried under vacuum, using a rotary evaporator below 40°C. The obtained crude ethanol extract of TCE was stored in the dark at −20°C for further studies.
2.2 Cell Culture
Mouse brain neuroblastoma cells (N2-A) and rat primary astrocytes (DI-TNC1) were purchased from American Type Culture Collection (ATCC, Manassas, VA). N2- A cell line used in the current investigation is a neuronal cell line known for its high lactate production compare to other cell lines. We, as well as others, have used this cell line and is considered an appropriate model to evaluate potential anti-cancer agents [22, 23]. We also used the N2-A cell line to investigate the “Warburg Effect” phenomenon [24], and cancer cells metabolism [25, 26]. On the other hand, the DI-TNC1 is an astrocyte immortal cell line with lower lactate efflux production compared to N2-A cells, an observation in our lab. The DI-TNC1 is very important in controlling brain energy metabolism [27, 28]. Cell culture Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin, DPBS, and trypsin were all from Atlanta Biologicals (Atlanta, GA, USA). Cells were cultured in 75-cm TC flask at 37°C in humidified 5% CO2 incubator and were subcultured as needed with trypsin/EDTA. Growing media was supplemented with 10% FBS (v/v), 4 mM L-glutamine, and 1% penicillin /streptomycin.
2.3 High Throughput Screening for Lactate Efflux Inhibition
For screening plant extracts as lactate efflux inhibitors, N2-A cells (5×104 /well) were seeded in 96-well plates and treated with 50 - 1000 μg/ml of plant ethanol extracts in a final volume 200 μl/well experimental media (phenol-free media supplemented with 1% each FBS/penicillin/streptomycin). Tested concentrations were determined based on previous preliminary studies. Control wells were treated only with ethanol at the highest used concentration (≤1.0%). After 4 h exposure period at 37°C and 5% CO2, 50 μl each of both experimental media and the lactate kit substrate mix were combined in another 96-well plate. The reaction was extended for 30 min at 37°C, CO2 - free incubator and stopped by 50 μl of 0.5 M acetic acid/well. The absorbance was measured at 490 nm using μQuant Monochromatic Microplate Spectrophotometer (BioTek, USA).
2.4 TCE Studies
2.4.1 Lactate efflux assay
As lactate efflux inhibitor, the effect of TCE was compared to standard MCT inhibitors, phloretin, and α-cyano-4-hydroxycinammic acid (CHC). Based on previous preliminary studies in our lab, N2-A cells were exposed to gradual concentrations between 0 to 250 μg/ml. All experiments were performed at least two separate times with n=4, and the control cells were exposed to the used solvents at the highest tested concentration (≤1.0% of ethanol for plant extract or 0.1 % DMSO for standard inhibitors). Blank wells without cells were also included in the test.
2.4.2 Cell viability assay
The redox dye resazurin was used for determining N2-A and DI-TNC1 cells viability after 24 h treatment with TCE at concentration range 0 – 250 μg/ml in experimental media. Control wells were treated only with ethanol at the highest used concentration (≤1.0%) and blank wells without cells were also involved in the test. In this assay, resazurin solution of 0.5 μg/ml in sterile phenol red free-phosphate-buffered saline (PBS) was used at concentration level 15% v/v. After an experimental period, the reduced resazurin was measured at 570 nm using μQuant Monochromatic Microplate Spectrophotometer (BioTek, USA). The percentage of N2-A cell survival compared to the control was calculated for IC50s determination.
2.4.3 Western blotting
Neuroblastoma cells were plated in 6 wells plate at concentration 106 cells/well and treated with low concentration of TCE (0-5 μg/ml) in the experimental media to keep cells alive and measure the changes in protein expression. Control wells were treated only with ethanol at the highest used concentration (0.1%) and blank wells without cells were also included in the test. After 4 h of incubation, cells were washed with PBS, pelleted and lysed for 30 minutes on ice with RIPA lysis buffer contains 1 X mammalian protease arrest. Samples were pulsed for few seconds with a probe sonicator and centrifuged at 10,000 ×g for 10 minutes at 4°C and the protein concentrations in cell lysates were determined using protein assay BCA. After that, the supernatant was diluted (1:1) with Laemmli sample buffer and boiled at 100°C for 3 minutes. Proteins from total cell lysates were loaded at consistent concentration 40 μg/ml and separated at 200 v constant voltages for 30-40 minutes using 10% SDS-PAGE gels and running buffer. Proteins were transferred to nitrocellulose membranes in the ice-cold transferring buffer for 90 minutes at 100 Voltage. Nitrocellulose membranes were incubated on a rocking shaker at room temperature for 1 hour with blocking buffer (5% non-fat dry milk in 1X PBST, pH 7.6) followed by 3x wash. All membranes were then incubated overnight with 10 ml of primary antibodies – diluted blocking buffer as following: MCT1 (1μg/ml); MCT3 (2.5 μg/ml); MCT4 (1:800); CD147 (1: 2,000) and GAPDH (1 μl/ml). After 3X wash with PBST, membranes were reincubated at RT for 3 hours with secondary antibody at dilution (1: 5,000). Finally, nitrocellulose membranes were washed with PBST and developed with chemiluminescence reagent. Images were captured using a Flour-S Max Multiimager (Bio-Rad Laboratories, Hercules, CA) and analyzed to obtain the band density with Quantity One Software (Bio-Rad Laboratories, Hercules, CA).
2.4.4 Caspase 3 apoptosis study
Apoptosis study was conducted by assessing caspase -3- activity using EnzChek® Caspase-3 assay kit. Briefly, N2-A cells were seeded at an initial concentration of 0.5 × 106 cell / well in 6 - well plates and treated with serial concentrations of TCE (0 - 30 μg/ml) in experimental media at a final volume of 3 ml/well. Tested concentrations were determined based on dose-response viability study. Control wells were treated only with ethanol at the highest used concentration (0.15 %) and blank wells without cells were also applied in the test. After 4 h incubation period, treated cells from each well were harvested, pelleted, washed in PBS. Cell pellets were resuspended in 50μL lysis buffer for 30 min on ice followed by centrifuge for 5 minutes at 4,100 ×g to pellet the debris. Lastly, 50 μl of each samples supernatant and the apoptosis kit substrate working solution were combined in another microplate well for 30 min at RT and the background fluorescence was determined by using 50 μL of the cell lysis buffer. Fluorescence intensity for each sample was measured (excitation/emission ~342/441 nm) using Synergy HTX Multi-Reader (BioTek, USA)
2.4.5 Acridine orange / ethidium bromide apoptosis study
Acridine orange/ ethidium bromide staining assay was performed to detect apoptotic changes in N2-A cells. The applied conditions for the assay were similar to the caspase-3 apoptosis study. Monolayer treated cells were washed 3X with PBS and incubated with the stain for 30 min. The dyes were added to the cells in 1:1 ratio at a final concentration of 5mg/mL acridine orange and 3 mg/ml of ethidium bromide. The excess dye was removed, and cells washed 2X with PBS and imaged at 40X magnification using Nikon Eclipse Ti fluorescence microscope (Nikon Instruments Inc., Melville, NY, USA).
2.4.6 Growth study and morphological changes
Cyto Scan™ water-soluble tetrazolium (WST 1) assay was used to measure growth rate in N2-A cells. Briefly, cells were plated at an initial density of 2 ×104 cells / well in 96 well plate and treated with TCE at concentration range (0 - 60 μg/ml) in a final volume 200 μl / well phenol-free growing media. The tested concentrations were determined based on dose-response viability study. Control cells were exposed to 0.3% ethanol in culture media and corresponding blanks were performed as treatments without cells. After 48 h of incubation, cells were combined with WST 1/CEC assay reagent at 10% v/v for 30 min to 4 h and the generated dark yellow-colored formazan was measured at 440 nm using Synergy HTX Multi-Reader (BioTek, USA). Cell density and morphological changes were photographed under phase - contrast inverted microscope Olympus 1 X 7I (Pittsburgh, PA, USA) at 20X magnification.
2.5 Statistical Analysis
Data were analyzed using the Graph Pad Prism 6.2 Software (San Diego, CA, USA). All data points were obtained from the average of at least two independent studies and expressed as mean ± SEM. Inhibitory concentrations (IC50s) for lactate efflux and cell viability studies and IG50 for growth inhibition studies, were determined by nonlinear regression with lowest 95% confidence interval and R2 best fit. The significance of the difference between two groups was determined by unpaired t-test, between control and treated groups using one-way ANOVA followed by Dunnett's multiple comparison's test. Significance of the difference between the control and treated groups is considered at *P = 0.05, ** P = 0.01, *** P = 0.001, and **** P = 0.0001.
3. RESULTS
3.1 High Throughput Plant Extracts Screening for Lactate Efflux Inhibitors
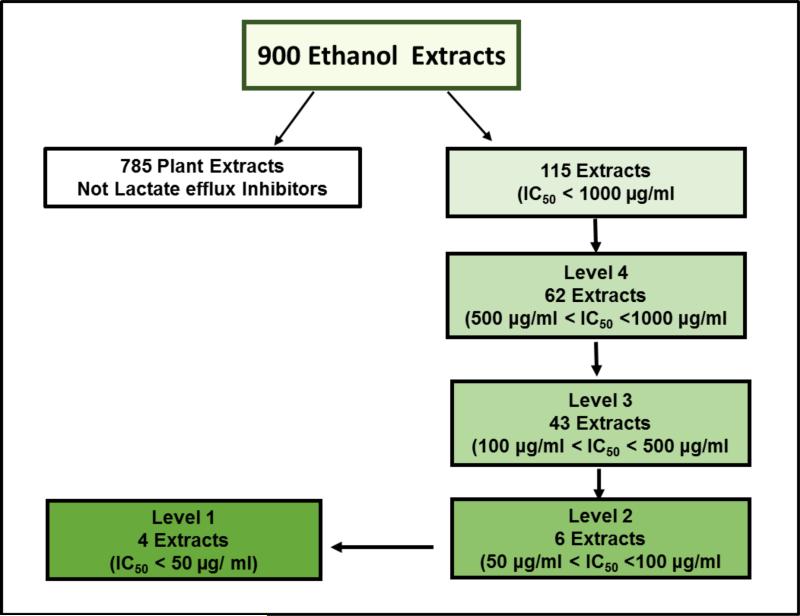
The high throughput screening of 900 ethanol plant extracts was designed to identify natural potent lactate efflux inhibitors in N2-A cancer cells at four tiers (Plant extract concentration: 50 - 1000 μg/ml). Based on < 50% lactate efflux compare to the control, 785 (87%) of the tested plant extracts were not active and excluded from the study after the first tier. The other extracts (115) were active and categorized according to their potency into four levels (Fig. 1 and Table 1). The fourth level were considered the least potent and included 62 extracts with (500 μg/ml < IC50 < 1000 μg/ml). 43 extracts showed average potency (100 μg/ml < IC50 < 500 μg/ml) and placed on the third level and 6 extracts showed higher potency (50 μg/ml < IC50 < 100 μg/ml) at the second level. Four plant extracts were categorized as the most potent at level 1 (IC50 < 50 μg/ml). These plant extracts were identified according to their potency as Terminalia chebula (IC50 42.78 μg/ ml), Bupleurum chinense (IC50 43.22 μg/ml), Trillium pendulum (IC50 49.82 μg/ml), and Rheum palmatum (IC50 49.82 μg/ml). Among these four extracts, Terminalia chebula was the most potent and therefore, further studies were performed using this plant extract.
Fig. 1. Schematic diagram of high throughput screening for 900-plant ethanol extracts (EE) to identify and rank natural lactate efflux inhibitors in N2-A cancer cells.
N2-A cellular lactate production of treated cells was compared to untreated normalized average % control total lactate production within 4 h of incubation with each extract. Extracts indicating an IC50 <1000 μg/ml were rescreened at lower concentrations (500, 100, and 50 μg/ml). According to the IC50s, the potent plant extracts were categorized into 4 levels, and 4 plant extracts were the most potent (IC50s < 50 μg/ml) and identified as Bupleurum chinense, Rheum palmatum, Terminalia chebula, and Trillium pendulum.
Table 1.
The effect of top ethanol plant extracts as lactate efflux inhibitors in N2-A cells. Cells were exposed 4h to different concentrations of the plant extracts. Compared to lactate production in control cells at the highest dose (1000 μg/ ml), 785-plant extracts were not active. The other plant extracts were categorized according to their potency as following: 62 extracts (500 μg/ml < IC50 < 1000 μg/ml) and ranked as the lease potent, 43 extracts (100 μg/ml < IC50 < 500 μg/ml), 6 extracts (50 μg/ml < IC50 < 100 μg/ml), and 4 ethanol plant extracts (IC50 < 50 μg/ml) and considered as the most potent.
| Rank | Common Name | Scientific Name |
|---|---|---|
| Level 1 (IC50 < 50 μg/ml) | ||
| Beth root | Trillium pendulum | |
| Bupleurum root | Bupleurum chinense | |
| Haritaki fruit | Terminalia chebula | |
| Turkey rhubarb root | Rheum palmatum | |
| Level 2 (50 μg/ml < IC50 < 100 μg/ml) | ||
| Green tea | Camellia sinensis | |
| Morning glory seeds | Semen pharbiditis | |
| Sancha leaf green tea | Camellia sinensis | |
| Thyme herb | Thymus vulgaris | |
| Witch hazel root | Hamamelis virginiana | |
| Yerba mate leaf | Ilex paraguarensis | |
| Level 3 (100 μg/ml < IC50 < 500 μg/ml) | ||
| Allspice | Pimenta dioica | |
| Babul chall bark | Acacia arabica | |
| Balm of gilead | Populus balsamifera L | |
| Bay leaf | Laurus nobilis | |
| Bayberry root bark | Morella cerifera | |
| Bhumy amalaki | Phyllanthus niruri | |
| Bilberry leaf | Vaccinium myrtillus | |
| Biota leaves | Biota orientalis | |
| Birch leaf | Betula alba | |
| Bishop's wort | Stachys officinales | |
| Blackberry leaf/root | Rubus fruticosus | |
| Buchu leaf | Agathosma betulina | |
| Buddleia flower bud | Buddleia officinalis | |
| Bushy knotweed rhizome | Polygonum cuspidatum | |
| Butternut bark | Juglans cinerea | |
| Canadian snake root | Assarum canadense | |
| Centaury herb, c/s | Centaurium erythracea | |
| Cleavers herb | Galium aparine | |
| Comfrey leaf | Symphytum officinale | |
| Dogbane leaf | Apocynum venetum | |
| Feverfew leaf and flower | Tanacetum parthenium | |
| Fleeceflower caulis | Polygonum multiflorum | |
| Fossilized teeth | Dens draconis | |
| Fringe bark tree | Chionanthus virginicus | |
| Golden eye-grass rhizome | Rhizoma curculiginis | |
| Gunpowder green tea | Camellia sinensis | |
| Heather flower | Calluna vulgaris | |
| Hyssop flowers | Hyssopus officinalis | |
| Italian spice herbal tea | Italian spice herbal tea | |
| jasmine flavored green tea | Jasminum officinale | |
| Lemon verbena leaf and flower | Aloysia triphylla | |
| Linden leaf | Tilia europaea | |
| Olive leaf | Olea europaea | |
| Osha root | Ligusticum porteri | |
| Paul D'Arko bark | Tabebuia impetiginosa | |
| Pipsissewa leaf | Chimaphila umbellata | |
| Pomegranate husk | Punica granatum | |
| Sassafras root bark | Sassafras albidum | |
| Soap horn thorn | Gleditsia sinensis | |
| Stone seeds | Lithospermum erythrorhizon | |
| White sage leaf | Salvia apiana | |
| Wild cherry bark | Prunus serotina | |
| Wild yam root | Dioscorea villosa | |
| Level 4 (500 μg/ml < IC50 < 1000 μg/ml) | ||
| Acanthopanax root bark | Acanthopanax gracilistylus | |
| Agrimony herb | Agrimonia eupatoria | |
| Akebia fruit | Fructus akebiae trifoliatae | |
| Alkanet root | Alkanna tinctoria | |
| Allspice berry powder | Pimenta dioica | |
| American pennyroyal herb | Hedeoma pulegioides | |
| Anise star seed and flower | Illicium verum | |
| Arjun bark | Terminalia arjuna | |
| Asafoetida, powder | Ferula assa-foetida | |
| Bian u herb | Polygonum aviculare | |
| Black cardamon pods | Fructus alpiniae oxyphyllae | |
| Black henna leaf | Lawsonia inermis | |
| Black pepper fruit | Piper nigrum | |
| Black walnut hull | Juglans nigra | |
| Blood root | Sanguinaria canadensis | |
| Blue verian arial portion | Verbena hastata | |
| Calamus root | Acorus calamus | |
| California poppy arial portion | Eschscholzia californica | |
| Cang Zhu | Atractylodes chinensis | |
| Carpesi fruit mult | Carpesium abrotanoides | |
| Celery seed | Apium graveolens | |
| Chang Shan (Hortensia) | Dichroa febrifuga | |
| Chaparral (greasewood) | Larrea tridentata | |
| Chili peppers flakes | Capsicum annuum | |
| Chinese Clematis Root | Radix clematidis | |
| Chinese thoroughwax | Bupleurum falcatum | |
| Cinnamon twig | Cinnamomum cassia | |
| Corriander seed powder | Coriandum sativum | |
| Cumin seed | Cuminum cyminum | |
| Desert thumb (red thumb) | Cynomorium songaricum | |
| Drgaon's blood | Dracaena cinnabari | |
| Epazote herb (wormseed) | Dysphania ambrosioides | |
| Eucalyptus leaf | Eucalyptus globulus | |
| Evergreen wisteria | Millettia reticulata | |
| Eyebright leaf and stem | Euphrasia officinalis | |
| Figwort herb | Scrophularia nodosa | |
| Fleece flower root | Polygonum multiflorum | |
| Frankincense | Boswellia resin | |
| Gallnut of Chinese sumac | Melaphis chinensis | |
| Galangal root | Alpinia galanga | |
| Gloryvine stem | Sargentodoxa cuneata | |
| Golden root | Rhodiola rosea | |
| Grapeseed extract | Vitis vinifera | |
| Hookweed roots | Cyathula officinalis root | |
| Indian lotus leaf | Nelumbo nucifera | |
| Irish breakfast green tea | Camellia sinensis | |
| Juniper berry, powder | Juniperus communis | |
| Kochia seed | Kochia scoparia | |
| Magnolia flower | Magnolia denudata | |
| Mandrake root | Podophyllum peltatum | |
| Marigold petals | Calendula officinalis | |
| Notopterygium root | Notopterygium incisium | |
| Nutmeg powder | Myristica fragans | |
| Orange powder | Citrus sinensis | |
| peppermint leaf | Mentha piperita | |
| Pipsissewa leaf | Chimaphila umbellata | |
| Plantain leaf | Plantago major | |
| Pomegranate Husk | Punicum granatum | |
| Red Henna leaf | Lawsonia inermis | |
| Sancha leaf green tea | Camellia sinensis | |
| Wood-fern, shield fern | Rhizoma dryopteris | |
| Yerba santa leaf | Eriodictyon californicum | |
3.2 TCE Lactate Efflux Inhibition Potency
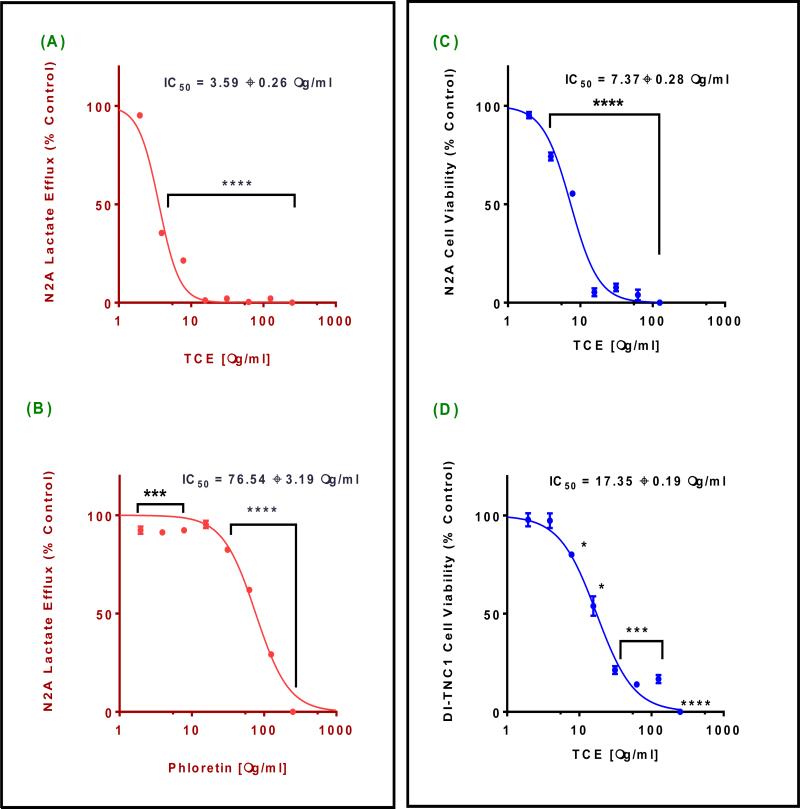
To determine TCE potency, we conducted dose-response studies for lactate efflux changes in N2-A cells supernatant. Lactate production was inversely proportional to the increased TCE concentrations. Inhibition of lactate efflux was highly significant (P = 0.0001), giving IC50 value of 3.59 ± 0.26 μg/ml (Fig. 2A). Lactate efflux inhibition was less than 10% in N2-A cells treated with a-cyano-4-hydroxycinammic acid (CHC), at the highest tested concentration (250 μg/ml = 1.32 mM). Meanwhile, phloretin induced highly significant effect (P< 0.0001) with IC50 76.54 ± 3.19 μg/ml (279.07 μM). Compare to the calculated IC50 of TCE, phloretin was less potent by 21.32 fold (Fig. 2B). Similarly, the dose - response of the cytotoxicity studies performed using N2-A cells vs. DI-TNC1 primary cells to assess the safety of TCE (Fig. 2 C and D). The data obtained indicated a significant inverse relationship between the viability and the tested concentrations in both cell lines (P = 0.0001). Noticeably, TCE was 2.35 fold less potent in the primary cells (IC50 of 17.35 ± 0.19 μg/ml) compare to N2-A cells (IC50 of 7.37 ± 0.28 μg/ml).
Fig. 2. Effect of Terminalia chebula (TCE) on lactate efflux and cell viability.
(A) and (B) are lactate production profile of N2-A cells after 4 h exposure to different concentrations of TCE and phloretin, respectively. (C) and (D) are cytotoxicity profile of N2-A and DI-TNC1 cells after 24 h exposure period to different concentrations of TCE. Statistical analysis of all studies was presented as the mean ± SEM from the average of two independent experiments, n=4 each. IC50s are average of two independent studies sigmoidal curves. The significance of the difference between controls vs. treated cells was determined using a one-way ANOVA followed by Dunnett's multiple comparisons test. Significance of difference between control and treatment is considered at *P = 0.05, *** P = 0.001, and **** P = 0.0001
3.3 TCE Reduces MCTs and CD147 Expression
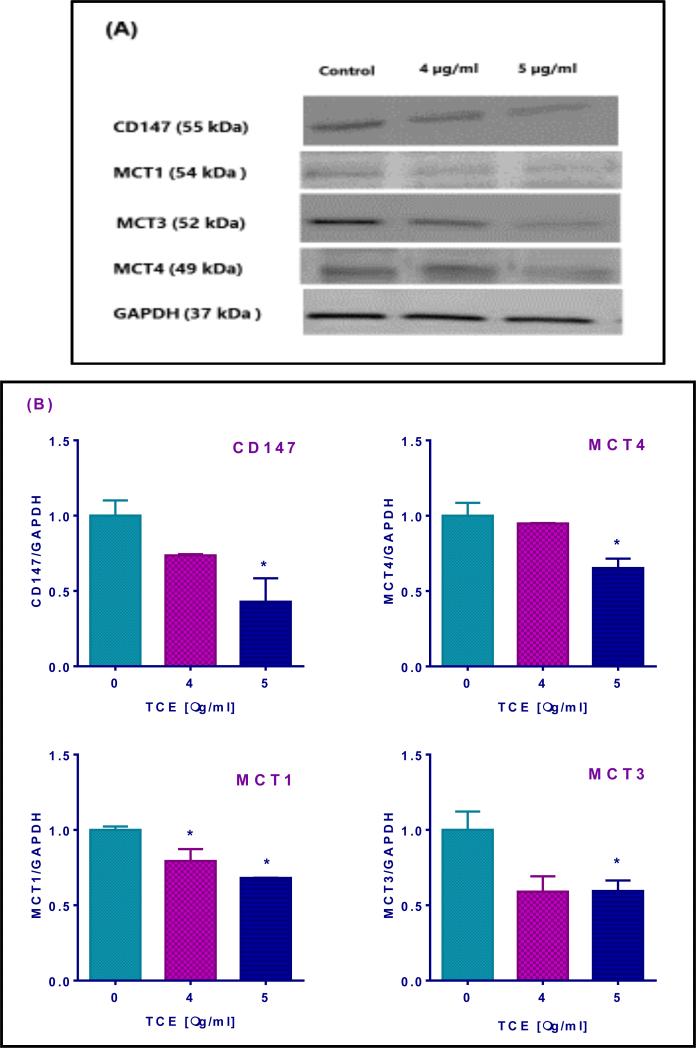
To understand the mode of action engaged in lactate efflux inhibition we performed Western blotting for N2-A cell lysates and evaluated protein expressions of monocarboxylate transporters and their chaperone CD147 after 4 h exposure to different concentrations of TCE. Antibodies detected the different MCTs, an indication of their presence in N2-A cell line (Fig. 3A). Moreover, at the highest tested dose 5 μg/ml, TCE-induced a significant decrease in protein expression (P = 0.05), giving 57% reduction in CD147; 35% reduction in MCT4 ; 32% reduction in MCT1; and 41% reduction in MCT3 expression (Fig. 3 B).
Fig. 3. Terminalia chebula extract (TCE) effect on the expression of monocarboxylate transporters (MCTs) and their chaperone CD147 in N2-A cancer cells after 4h treatment with concentration range 0 to 5 μg/ml of TCE.
(A) Indicates the presence of all candidates as detected by their molecular weight compared to the standard protein. The decrease in band intensities appeared precisely at 5 μg/ml, and loading consistency was confirmed by GAPDH. (B) Data obtained from two independent studies showed a significant decrease in protein expression in all candidates at 5 μg/ml. Statistical analysis was presented as the mean SD from the average of two independent experiments. The significance of the difference between the control and treated cell lysates was determined using one-way ANOVA followed by Dunnett's multiple comparisons tests. The significance level was set at *P = 0.05.
3.4 TCE Induces Apoptosis, Morphological Changes, and Activates Caspase 3 in N2-A Cells
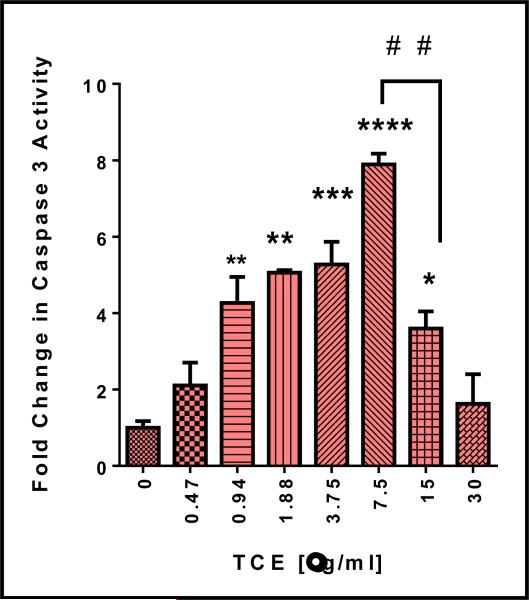
The change of caspases 3 activity was used as a marker for apoptosis and cell death that might be attributed to lactate efflux inhibition. Cell apoptosis was measured in N2-A cells after 4 h exposure to TCE. The results show that a significant increase in caspase 3 activity, in a dose - dependent manner, was detected in the cell lysates (Fig. 4). The significant difference between treated and control cells was detected at 7.5 μg/ml (P = 0.0001), giving almost 8 folds’ increase in caspase activity relative to the control cells. Also, a significant decrease was also obtained (# # P = 0.01) at a higher dose (15 μg/ml).
Fig. 4. Activation of caspase 3 in N2-A cells by Terminalia chebula (TCE).
Caspase 3 was measured in the cell lysates of two independent studies with n=3 and expressed as fold increase compares to the control. The significance of the difference between treated cells vs. control. Significance is considered at * P = 0.05, ** P = 0.01, *** P = 0.001, **** P = 0.0001, and # # P = 0.01.
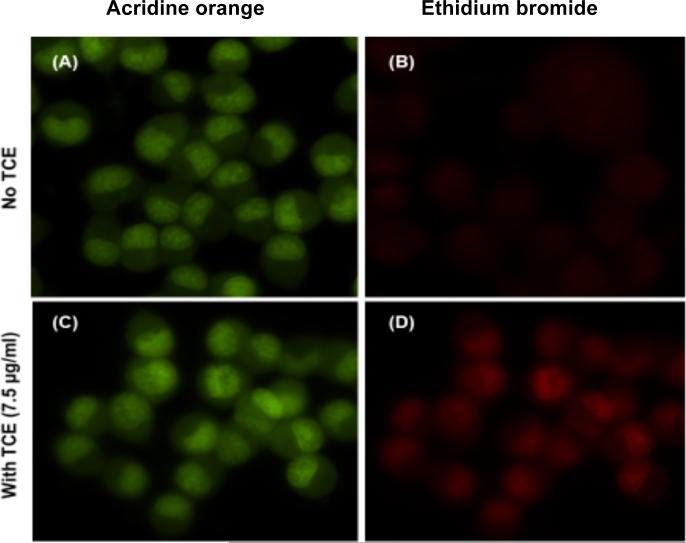
The apoptosis-related morphological changes of TCE were further investigated using acridine orange/ethidium bromide fluorescence assay. Untreated cells appeared with uniformly green nuclei (Fig. 5 A) while different degrees of early and late apoptotic features appeared clearly in cells treated with 7.5 μg/ml (Fig. 5 C and D). Early apoptotic cells appeared with bright green dots in the nuclei, while chromatin condensation and nuclear fragmentation were detected in the late apoptotic stage as cells lose the membrane integrity and incorporate a red color - ethidium bromide.
Fig. 5. Apoptotic effect of Terminalia chebula (TCE) in N2-A cells.
(A) Control cells stained with acridine orange and appeared with uniform green - stained nuclei. (B) Control cells stained with ethidium bromide. (C) Acridine orange - stained cells treated for 4 h with 7.5 μg/ml of TCE appeared with bright dots at the nuclei as symptoms of early apoptosis. (D) Ethidium bromide stained cells treated for 4 h with 7.5 μg/ml of TCE appeared red color and fragmented and condensed nuclei were detected in late apoptotic cells. Microscopic magnification was 40X.
3.5 The Growth Inhibition Effects of TCE
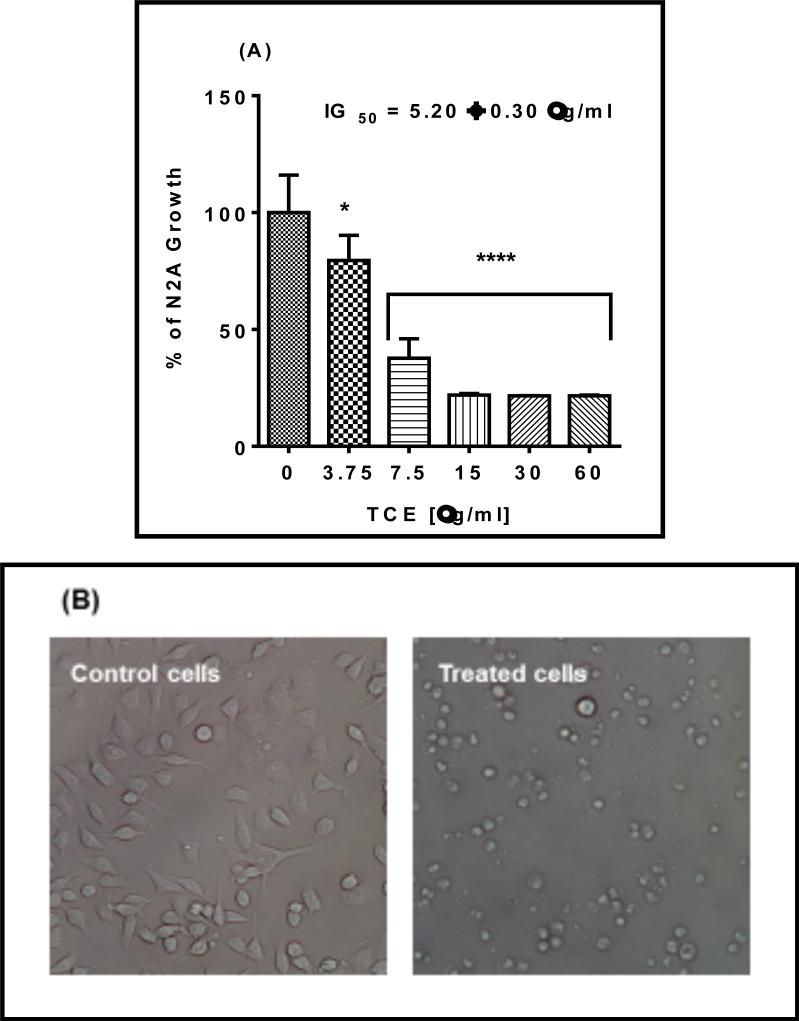
The impact of TCE on N2-A cell growth was evaluated at 48 h exposure period. TCE decreased cell proliferation in a dose-dependent pattern with a highly significant reduction in cell proliferation (P = 0.0001) was observed at the tested concentration of 7.5 μg/ml and above, giving IG50 = 5.2 ± 0.30 μg/ml (Fig. 6A). Remarkably, almost 76% reduction in cell proliferation was obtained at 15 μg/ml of TCE and remained consistent at the other higher doses. Also, Phase-contrast microscopy revealed that treated cells decreased in numbers and appeared round with shrunk size compared to the control. (Fig. 6B).
Fig. 6. Effect of Terminalia chebula (TCE) on N-2A cell growth and morphology.
(A). Cell growth activity of N2-A treated for 48h with different concentrations of TCE. Statistical analysis is presented as the mean ± SEM of two independent experiments with n=4. The significance of the difference between treated cells vs. control was determined using one-way ANOVA followed by Dunnett's multiple comparisons test. The IG50 is the average of two studies sigmoidal curves. Significance is considered at *P = 0.05, and **** P = 0.0001. (B). Phase contrast of N2-A cells treated for 48 h with or without 15.0 μg/ml of TCE and microscope magnification was 20 x objective magnification.
4. DISCUSSION
Lactate efflux is critical for cancer cell metabolism and proliferation. Thus, targeting lactate produced by cancer cells was the primary goal of this study. Extracts of 900 plants were screened for lactate efflux inhibition in N2-A neuroblastoma cells that are characterized by a high metabolic rate and excess lactate efflux [29]. The extract of Terminalia chebula (TCE) plant was the most potent extract as lactate efflux inhibitor. The plant, Terminalia chebula Retz, belongs to the family Combretaceae and also called black Myrobalans (English) and Harad (Hindi). The full grown plant is a tall tree up to 80 feet in height, is native to India, known as the ‘King of Medicine’ since it was used in healing many diseases such as heart diseases, asthma, gout, bleeding piles, vomiting, diarrhea, ulcers, sore throat, and dysentery [17]. The extensively studied Terminalia species indicate that this plant has a wide spectrum of medicinal effects. The plant was reported to have an antimicrobial [30], antiviral, antimalarial and antifungal [31], antiprotozoal [32], anti-inflammatory, anti-arthritic [33], antidiabetic [34], hepatoprotective [35], antioxidant [36], antianaphylactic [37], antimutagenic [38], and anticancer [39-43] effects. Several studies have also indicated that the methanolic and water extracts of TCE have an inhibitory action on the human immunodeficiency virus [44] and immunomodulatory action [45]. Additionally, a recent study using the rat pheochromocytoma (PC12) cell line indicated that the extract of the dried ripe fruit has a neuroprotective effect against ischemia related damage [46]. Several in vivo studies on the pharmacological effects of the extract of the Terminalia chebula plant (TCE) were investigated using the rat and the mouse. Many reports indicated the effectiveness of this plant extract as an anti-inflammatory agent [47-48]. Moreover, the chemopreventive effects of TCE in stomach cancer in the rat were reported earlier [49].
Since our primary concern in this study is to evaluate the levels of extracellular lactate as an indication of functional MCTs, we examined the potency of TCE comparing to the well- known lactate inhibitors phloretin and CHC [50, 51]. The obtained results indicate that 50% of lactate efflux inhibition in N2-A cell was obtained when cells were treated with 279.07 μM of phloretin. The obtained results are in agreement with the previously reported study that found 300 μM of phloretin inhibited lactate transport in erythrocytes [52]. Interestingly, our data showed a remarkable effect of TCE over phloretin. On the contrary, current data did not show a significant inhibitory effect of CHC at the highest tested concentration. In spite of the reported effects of CHC as an MCT1 selective inhibitor [53] by affecting the expression of MCT1 [3], no sufficient information about the impact of CHC on N2-A cells. However, our results agree with previous studies that 5mM of CHC did not inhibit lactate efflux in glial cells [54] and should be at least 10 mM to inhibit MCT efflux in malignant gliomas [55].
Current literature did not report the selective cytotoxicity of TCE among different cancer cell lines. However, Terminalia chebula was reported as a safe chemopreventive drug within the recommended Ayurvedic specifications [56]. Also, in an in vivo study, Terminalia chebula dried fruits water extract was found to cause neither acute nor chronic toxicities when tested in male or female rats [57]. These data agree with our cytotoxicity study on DI-TNC1 primary cell line.
To explore the mechanism of action of lactate efflux inhibition by TCE, we examined MCT transporters as important pH regulators in high glycolytic solid tumors that mediate lactate transportation across the plasma membranes [58]. Also, the suppression of monocarboxylate transporters is considered the first step in apoptosis [59]. Lactate efflux through MCT4 was previously reported [2]. However, MCT1 and MCT3 might facilitate lactate passing through the plasma membrane under certain conditions [16-17]. On the contrary, MCT2 expression is reduced in highly glycolytic cancer cells [60] since it involves in lactate uptake under normal metabolism [61]. Thus, Western blotting was performed to evaluate the expression of MCT1, MCT3, and MCT4 in treated N2-A cells. Furthermore, the expression of a chaperone to some MCTs was also studied. CD147 is a multifunctional protein and also known as basigin, controlling and regulating energy metabolism of cancer cells [62]. Importantly, it is necessary for MCTs stabilization and expression at the cell membrane [63]. Accordingly, disabling MCTs through disrupting their association with CD147 is considered one of the novel approaches to inhibiting MCTs.
To our knowledge, this is the first study to report on the expression of MCT1, MCT3, and MCT4 and the chaperone CD147 in neuroblastoma N2-A cells. However, previous studies found similar expression of MCT1 in human neuroblastoma cell lines (IMR32, NGP, and SK-N-SH) [29] and MCT4 expression was higher in MDA-MB-231 [64]. Although all proteins under investigation showed a significant decrease in their expression at the highest tested dose of TCE, the highest reduction was observed in CD147 expression. Considering all these findings, we might attribute TCE inhibition of lactate efflux to the reduction of CD147 expression more than MCT4 itself. In other words, TCE may have inhibited MCT4 function indirectly through CD147 suppression. The role of MCT3 in cancer cells is poorly studied. However, a previous study on the retina of the rat reported MCT3 as lactate efflux transporter [65]. Interestingly, the decrease in MCT1 expression might be another reason for the insignificant lactate efflux inhibitory effect of CHC in N2-A cells, an interpretation that agrees with a previous study since CHC exerts an inhibitory effect on tumors cells expressing MCT1 at the plasma membrane [15].
In the current study, apoptotic effect of TCE was confirmed by caspase 3 activity. Caspase 3 is a cysteine protease, and its activation is considered a critical step in cell apoptosis [66]. Our findings are in agreement with earlier studies indicated that quercetin isolated from the fruits of Terminalia spp was found to induce apoptotic effects in N2-A cells [67], chebulagic acid was also reported to induce apoptosis in COLO-205 cells [68]. Similarly, apoptosis was reported in human breast cancer MDA-MB-231 treated with pentagalloylglucose and quercetin [69] and HL-60 cells treated with ellagitannins [70]. Current proliferation study was comparable to the previous study that showed a decrease in cell proliferation upon lactate efflux inhibition in breast cancer cells [71]. Despite the differences in the method of extraction, as well as the cell line, the growth inhibition effect was profound by Terminalia chebula when tested in various cell lines [39].
5. CONCLUSION
Out of 900 ethanol plant extracts screened, Terminalia chebula ethanol extract was found to be the most potent lactate efflux inhibitor with the ability to inhibit chaperone CD147 expression and impact the function of monocarboxylate transporters. Furthermore, TCE has growth inhibition and apoptotic effects. The obtained results indicate that the plant Terminalia chebula constituent(s) may contain new targets for the management of neuroblastoma.
Footnotes
Authors’ contributions:
This work was carried out in collaboration between all authors. Authors SSM, NOZ, MGK and KFAS designed the study, performed the statistical analysis, wrote the protocol and wrote the first draft of the manuscript. Author ET participated in protein expression study. Authors SGG and GRS managed the literature searches. All authors read and approved the final manuscript.
CONSENT: Not Applicable
COMPETING INTERESTS
Authors have declared that no competing interests exist.
REFERENCES
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Draoui N, Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. 2011;4(6):727–32. doi: 10.1242/dmm.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10(11):1772–83. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996;14(2):176–86. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 5.Schlappack OK, Zimmermann A, Hill RP. Glucose starvation and acidosis: effect on experimental metastatic potential, DNA content and MTX resistance of murine tumour cells. Br J Cancer. 1991;64(4):663–70. doi: 10.1038/bjc.1991.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–54. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 7.Izumi H, Takahashi M, Uramoto H, Nakayama Y, Oyama T, Wang KY, et al. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci. 2011;102(5):1007–13. doi: 10.1111/j.1349-7006.2011.01908.x. [DOI] [PubMed] [Google Scholar]
- 8.Thammasit P, Sangboonruang S, Suwanpairoj S, Khamaikawin W, Intasai N, Kasinrerk W, et al. Intracellular Acidosis Promotes Mitochondrial Apoptosis Pathway: Role of EMMPRIN Down-regulation via Specific Single-chain Fv Intrabody. J Cancer. 2015;6(3):276–86. doi: 10.7150/jca.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol. 2000;2(6):318–25. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- 10.Barry MA, Eastman A. Endonuclease activation during apoptosis: the role of cytosolic Ca2+ and pH. Biochem Biophys Res Commun. 1992;186(2):782–9. doi: 10.1016/0006-291x(92)90814-2. [DOI] [PubMed] [Google Scholar]
- 11.Park HJ, Lyons JC, Ohtsubo T, Song CW. Acidic environment causes apoptosis by increasing caspase activity. Br J Cancer. 1999;80(12):1892–7. doi: 10.1038/sj.bjc.6690617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grinstein S, Rotin D, Mason MJ. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989;988(1):73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- 13.Gillies RJ, Martinez-Zaguilan R, Martinez GM, Serrano R, Perona R. Tumorigenic 3T3 cells maintain an alkaline intracellular pH under physiological conditions. Proc Natl Acad Sci U S A. 1990;87(19):7414–8. doi: 10.1073/pnas.87.19.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halestrap AP. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life. 2012;64(1):1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 15.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118(12):3930–42. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343(Pt 2):281–99. [PMC free article] [PubMed] [Google Scholar]
- 17.Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F. Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr. 2012;44(1):127–39. doi: 10.1007/s10863-012-9428-1. [DOI] [PubMed] [Google Scholar]
- 18.Sankara Aditya J, Kumar L. Naresh. Animisha Mokkapati. vitro anti-cancer activities of few plant extracts against MCF-7 and HT-29 cell lines VSPK. Int. J. Pharm. Sci. 2013;3(2):185–188. [Google Scholar]
- 19.Shim CK, Cheon EP, Kang KW, Seo KS, Han HK. Inhibition effect of flavonoids on monocarboxylate transporter 1 (MCT1) in Caco-2 cells. J Pharm Pharmacol. 2007;59(11):1515–9. doi: 10.1211/jpp.59.11.0008. [DOI] [PubMed] [Google Scholar]
- 20.Baltazar F, Pinheiro C, Morais-Santos F, Azevedo-Silva J, Queiros O, Preto A, et al. Monocarboxylate transporters as targets and mediators in cancer therapy response. Histol Histopathol. 2014;29(12):1511–24. doi: 10.14670/HH-29.1511. [DOI] [PubMed] [Google Scholar]
- 21.Cieniak C, Walshe-Roussel B, Liu R, Muhammad A, Saleem A, Haddad PS, et al. Phytochemical Comparison of the Water and Ethanol Leaf Extracts of the Cree medicinal plant, Sarracenia purpurea L. (Sarraceniaceae). J Pharm Pharm Sci. 2015;18(4):484–93. doi: 10.18433/j35w27. [DOI] [PubMed] [Google Scholar]
- 22.Mazzio EA, Soliman KF. In vitro screening of tumoricidal properties of international medicinal herbs: part II. Phytother Res. 2010;24(12):1813–24. doi: 10.1002/ptr.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finklestein JZ, Tittle K, Meshnik R, Weiner J. Murine neuroblastoma: further evaluation of the C1300 model with single antitumor agents. Cancer Chemother Rep. 1975;59(5):975–83. [PubMed] [Google Scholar]
- 24.Mazzio EA, Boukli N, Rivera N, Soliman KF. Pericellular pH homeostasis is a primary function of the Warburg effect: inversion of metabolic systems to control lactate steady state in tumor cells. Cancer Sci. 2012;103(3):422–32. doi: 10.1111/j.1349-7006.2012.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzio EA, Smith B, Soliman KF. Evaluation of endogenous acidic metabolic products associated with carbohydrate metabolism in tumor cells. Cell Biol Toxicol. 2010;26(3):177–88. doi: 10.1007/s10565-009-9138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzio E, Soliman KF. Whole genome expression profile in neuroblastoma cells exposed to 1-methyl-4-phenylpyridine. Neurotoxicology. 2012;33(5):1156–69. doi: 10.1016/j.neuro.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354(1387):1155–63. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellerin L, Stolz M, Sorg O, Martin JL, Deschepper CF, Magistretti PJ. Regulation of energy metabolism by neurotransmitters in astrocytes in primary culture and in an immortalized cell line. Glia. 1997;21(1):74–83. doi: 10.1002/(sici)1098-1136(199709)21:1<74::aid-glia8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Fang J, Quinones QJ, Holman TL, Morowitz MJ, Wang Q, Zhao H, et al. The H+-linked monocarboxylate transporter (MCT1/SLC16A1): a potential therapeutic target for high-risk neuroblastoma. Mol Pharmacol. 2006;70(6):2108–15. doi: 10.1124/mol.106.026245. [DOI] [PubMed] [Google Scholar]
- 30.Khan AA, Kumar V, Singh BK, Singh R. Evaluation of wound healing property of Terminalia catappa on excision wound models in Wistar rats. Drug Res (Stuttg) 2014;64(5):225–8. doi: 10.1055/s-0033-1357203. [DOI] [PubMed] [Google Scholar]
- 31.Valsaraj R, Pushpangadan P, Smitt UW, Adsersen A, Christensen SB, Sittie A, et al. New anti-HIV-1, antimalarial, and antifungal compounds from Terminalia bellerica. J Nat Prod. 1997;60(7):739–42. doi: 10.1021/np970010m. [DOI] [PubMed] [Google Scholar]
- 32.Bagavan A, Rahuman AA. Evaluation of larvicidal activity of medicinal plant extracts against three mosquito vectors. Asian Pac J Trop Med. 2011;4(1):29–34. doi: 10.1016/S1995-7645(11)60027-8. [DOI] [PubMed] [Google Scholar]
- 33.Nair V, Singh S, Gupta YK. Anti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental models. J Pharm Pharmacol. 2010;62(12):1801–6. doi: 10.1111/j.2042-7158.2010.01193.x. [DOI] [PubMed] [Google Scholar]
- 34.Huang YN, Zhao DD, Gao B, Zhong K, Zhu RX, Zhang Y, et al. Anti-hyperglycemic effect of chebulagic acid from the fruits of Terminalia chebula Retz. Int J Mol Sci. 2012;13(5):6320–33. doi: 10.3390/ijms13056320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HS, Jung SH, Yun BS, Lee KW. Isolation of chebulic acid from Terminalia chebula Retz. and its antioxidant effect in isolated rat hepatocytes. Arch Toxicol. 2007;81(3):211–8. doi: 10.1007/s00204-006-0139-4. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Sun F, Ma L, Wang J, Qin H, Du G. In vitro evaluation of the antioxidant capacity of triethylchebulate, an aglycone from Terminalia chebula Retz fruit. Indian J Pharmacol. 2011;43(3):320–3. doi: 10.4103/0253-7613.81508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin TY, Jeong HJ, Kim DK, Kim SH, Lee JK, Kim DK, et al. Inhibitory action of water soluble fraction of Terminalia chebula on systemic and local anaphylaxis. J Ethnopharmacol. 2001;74(2):133–40. doi: 10.1016/s0378-8741(00)00360-3. [DOI] [PubMed] [Google Scholar]
- 38.Kaur S, Grover IS, Singh M, Kaur S. Antimutagenicity of hydrolyzable tannins from Terminalia chebula in Salmonella typhimurium. Mutat Res. 1998;419(1-3):169–79. doi: 10.1016/s1383-5718(98)00130-2. [DOI] [PubMed] [Google Scholar]
- 39.Saleem A, Husheem M, Harkonen P, Pihlaja K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J Ethnopharmacol. 2002;81(3):327–36. doi: 10.1016/s0378-8741(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 40.Hartwell JL. Plants used against cancer: a survey. 1984 [Google Scholar]
- 41.Tagne RS, Telefo BP, Nyemb JN, Yemele DM, Njina SN, Goka SM, et al. Anticancer and antioxidant activities of methanol extracts and fractions of some Cameroonian medicinal plants. Asian Pac J Trop Med. 2014;7s1:S442–7. doi: 10.1016/S1995-7645(14)60272-8. [DOI] [PubMed] [Google Scholar]
- 42.Kumar N, Gangappa D, Gupta G, Karnati R. Chebulagic acid from Terminalia chebula causes G1 arrest, inhibits NFkappaB and induces apoptosis in retinoblastoma cells. BMC Complement Altern Med. 2014;14:319. doi: 10.1186/1472-6882-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghate NB, Hazra B, Sarkar R, Chaudhuri D, Mandal N. Alteration of Bax/Bcl-2 ratio contributes to Terminalia belerica-induced apoptosis in human lung and breast carcinoma. In Vitro Cell Dev Biol Anim. 2014;50(6):527–37. doi: 10.1007/s11626-013-9726-x. [DOI] [PubMed] [Google Scholar]
- 44.el-Mekkawy S, Meselhy MR, Kusumoto IT, Kadota S, Hattori M, Namba T. Inhibitory effects of Egyptian folk medicines on human immunodeficiency virus (HIV) reverse transcriptase. Chem Pharm Bull (Tokyo) 1995;43(4):641–8. doi: 10.1248/cpb.43.641. [DOI] [PubMed] [Google Scholar]
- 45.Belapurkar P, Goyal P, Tiwari-Barua P. Immunomodulatory effects of Triphala and its individual constituents: a review. Indian J Pharm Sci. 2014;76(6):467–75. [PMC free article] [PubMed] [Google Scholar]
- 46.Gaire BP, Kim H. Neuroprotective effects of Fructus Chebulae extracts on experimental models of cerebral ischemia. J Tradit Chin Med. 2014;34(1):69–75. doi: 10.1016/s0254-6272(14)60057-1. [DOI] [PubMed] [Google Scholar]
- 47.Bag A, Kumar Bhattacharyya S, Kumar Pal N, Ranjan Chattopadhyay R. Anti-inflammatory, anti-lipid peroxidative, antioxidant and membrane stabilizing activities of hydroalcoholic extract of Terminalia chebula fruits. Pharm Biol. 2013;51(12):1515–20. doi: 10.3109/13880209.2013.799709. [DOI] [PubMed] [Google Scholar]
- 48.Kalaiselvan S, Rasool MK. The anti-inflammatory effect of Triphala in arthritic-induced rats. Pharm Biol. 2015;53(1):51–60. doi: 10.3109/13880209.2014.910237. [DOI] [PubMed] [Google Scholar]
- 49.Deep G, Dhiman M, Rao AR, Kale RK. Chemopreventive potential of Triphala (a composite Indian drug) on benzo(a)pyrene-induced forestomach tumorigenesis in murine tumor model system. J Exp Clin Cancer Res. 2005;24(4):555–63. [PubMed] [Google Scholar]
- 50.Wang X, Poole RC, Halestrap AP, Levi AJ. Characterization of the inhibition by stilbene disulphonates and phloretin of lactate and pyruvate transport into rat and guinea-pig cardiac myocytes suggests the presence of two kinetically distinct carriers in heart cells. Biochem J. 1993;290(Pt 1):249–58. doi: 10.1042/bj2900249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colen CB, Shen Y, Ghoddoussi F, Yu P, Francis TB, Koch BJ, et al. Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo study. Neoplasia. 2011;13(7):620–32. doi: 10.1593/neo.11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deuticke B, Rickert I, Beyer E. Stereoselective, SH-dependent transfer of lactate in mammalian erythrocytes. Biochim Biophys Acta. 1978;507(1):137–55. doi: 10.1016/0005-2736(78)90381-4. [DOI] [PubMed] [Google Scholar]
- 53.Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7(3):e33418. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volk C, Kempski B, Kempski OS. Inhibition of lactate export by quercetin acidifies rat glial cells in vitro. Neurosci Lett. 1997;223(2):121–4. doi: 10.1016/s0304-3940(97)13420-6. [DOI] [PubMed] [Google Scholar]
- 55.Colen CB, Seraji-Bozorgzad N, Marples B, Galloway MP, Sloan AE, Mathupala SP. Metabolic remodeling of malignant gliomas for enhanced sensitization during radiotherapy: an in vitro study. Neurosurgery. 2006;59(6):1313–23. doi: 10.1227/01.NEU.0000249218.65332.BF. discussion 1323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pellati F, Bruni R, Righi D, Grandini A, Tognolini M, Pio Prencipe F, et al. Metabolite profiling of polyphenols in a Terminalia chebula Retzius ayurvedic decoction and evaluation of its chemopreventive activity. J Ethnopharmacol. 2013;147(2):277–85. doi: 10.1016/j.jep.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 57.Sireeratawong S, Jaijoy K, Panunto W, Nanna U, Lertprasertsuke N, Soonthornchareonnon N. Acute and chronic toxicity studies of the water extract from dried fruits of Terminalia bellerica (Gaertn.) Roxb. In Sprague-Dawley rats. Afr J Tradit Complement Altern Med. 2013;10(2):223–31. doi: 10.4314/ajtcam.v10i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindinger MI, Leung MJ, Hawke TJ. Inward flux of lactate(-) through monocarboxylate transporters contributes to the regulatory volume increase in mouse muscle fibers. PLoS One. 2013;8(12):e84451. doi: 10.1371/journal.pone.0084451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moley KH, Mueckler MM. Glucose transport and apoptosis. Apoptosis. 2000;5(2):99–105. doi: 10.1023/a:1009697908332. [DOI] [PubMed] [Google Scholar]
- 60.Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, et al. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008;452(2):139–46. doi: 10.1007/s00428-007-0558-5. [DOI] [PubMed] [Google Scholar]
- 61.Broer S, Broer A, Schneider HP, Stegen C, Halestrap AP, Deitmer JW. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J. 1999;341(Pt 3):529–35. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchiq I, Albrengues J, Granja S, Gaggioli C, Pouyssegur J, Simon MP. Knock out of the BASIGIN/CD147 chaperone of lactate/H+ symporters disproves its pro-tumour action via extracellular matrix metalloproteases (MMPs) induction. Oncotarget. 2015;6(28):24636–48. doi: 10.18632/oncotarget.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. Embo j. 2000;19(15):3896–904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hussien R, Brooks GA. Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol Genomics. 2011;43(5):255–64. doi: 10.1152/physiolgenomics.00177.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72(19):5130–40. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 66.Zimmermann KC, Green DR. How cells die: apoptosis pathways. J Allergy Clin Immunol. 2001;108(4 Suppl):S99–103. doi: 10.1067/mai.2001.117819. [DOI] [PubMed] [Google Scholar]
- 67.Sugantha Priya E, Selvakumar K, Bavithra S, Elumalai P, Arunkumar R, Raja Singh P, et al. Anti-cancer activity of quercetin in neuroblastoma: an in vitro approach. Neurol Sci. 2014;35(2):163–70. doi: 10.1007/s10072-013-1462-1. [DOI] [PubMed] [Google Scholar]
- 68.Reddy DB, Reddy TC, Jyotsna G, Sharan S, Priya N, Lakshmipathi V, et al. Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J Ethnopharmacol. 2009;124(3):506–12. doi: 10.1016/j.jep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 69.Huang C, Lee SY, Lin CL, Tu TH, Chen LH, Chen YJ, et al. Co-treatment with quercetin and 1,2,3,4,6-penta-O-galloyl-beta-D-glucose causes cell cycle arrest and apoptosis in human breast cancer MDA-MB-231 and AU565 cells. J Agric Food Chem. 2013;61(26):6430–45. doi: 10.1021/jf305253m. [DOI] [PubMed] [Google Scholar]
- 70.Chen LG, Huang WT, Lee LT, Wang CC. Ellagitannins from Terminalia calamansanai induced apoptosis in HL-60 cells. Toxicol In Vitro. 2009;23(4):603–9. doi: 10.1016/j.tiv.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 71.Morais-Santos F, Miranda-Gonçalves V, Pinheiro S, Vieira AF, Paredes J, Schmitt FC, et al. Differential sensitivities to lactate transport inhibitors of breast cancer cell lines. Endocrine-related cancer. 2014;21(1):27–38. doi: 10.1530/ERC-13-0132. [DOI] [PubMed] [Google Scholar]