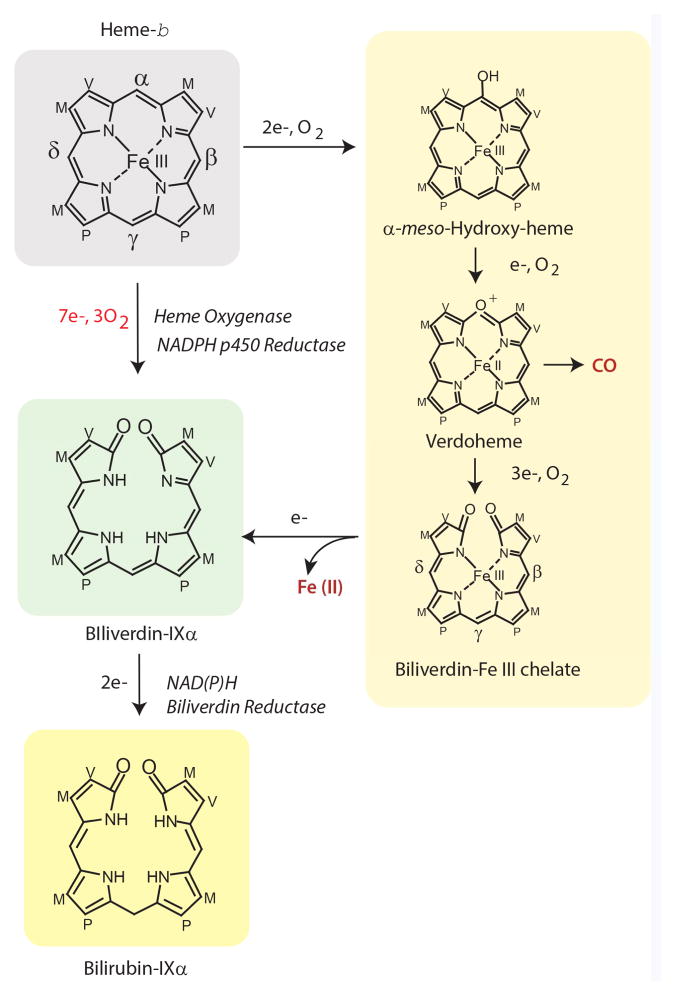
Figure 1.
The heme oxygenase (HO) reaction cleaves heme at the α-methene bridge carbon and generates carbon monoxide (CO), biliverdin-IXα. and ferrous iron (Fe II). The reaction proceeds through three sequential oxidation steps each requiring one mole of molecular oxygen (O2), and a total of seven electrons from NADPH: cytochrome p450 reductase. Three reaction intermediates have been proposed: α-meso-hydroxyheme, verdoheme, and the Fe (III)-biliverdin complex. Upon univalent reduction, the Fe (III)-biliverdin complex dissociates to form biliverdin-IXα and free Fe (II). The completion of enzymatic heme degradation involves the divalent reduction of biliverdin-IXα by NAD(P)H: biliverdin reductase (BVR; E.C. 1.3.1.24), which produces the lipid soluble pigment bilirubin-IXα. Heme side chains are designated: M=Methyl, V=Vinyl, P=Propionate.