Abstract
Intracranial neoplasia is a common clinical condition in domestic companion animals, particularly in dogs. Application of advances in standard diagnostic and therapeutic modalities together with a broad interest in the development of novel translational therapeutic strategies in dogs has resulted in clinically relevant improvements in outcome for many canine patients. This review highlights the status of current diagnostic and therapeutic approaches to intracranial neoplasia and areas of novel treatment currently in development.
Keywords: Brain tumor, CNS disorders, Neuroimaging, Neurology, Neurosurgery, Oncology, Oncology treatment
Intracranial neoplasia represents a major cause of morbidity and mortality in companion animals, predominantly in dogs. Recent advances in diagnosis and treatment of intracranial neoplasia in veterinary medicine have been driven by a combination of improved access to advanced imaging and neurosurgical equipment together with the recognition of brain tumors in dogs as a viable and potentially valuable model for translational and basic research.1, 2, 3 Collaborative therapeutic research embracing the “one medicine” approach has demanded both basic molecular genetic characterization of these tumors in dogs to validate the next generation of targeted therapies, and advances in the diagnostic and surgical techniques necessary to apply these approaches in a clinical setting. Accurate data for true incidence of brain tumors in dogs are limited to a study in the 1960s and 1970s in Northern California4, 5; however, the reported 14.5 cases per 100,000 dogs included all nervous system tumors, and numbers were small. These results are similar to data in humans where an incidence of 20.59 primary CNS tumors per 100,000 human patients in the United States has been reported.6 A more accurate comparison may be based on necropsy data where intracranial/nervous system neoplasia has been reported in approximately 2–4.5% of dogs7, 8, 9 compared to approximately 2% of human patients.10 Given the practical limitations of veterinary care, it is likely that true incidence of brain tumors in dogs has been underestimated. Although individual studies vary, meningiomas compromise approximately 50% of primary tumors in dogs with gliomas representing 40–70% and choroid plexus tumors being the next most common tumor.7, 9, 11, 12 Secondary neoplasia accounts for approximately 50% of all intracranial tumors in dogs, with the most common tumor types being hemangiosarcoma, pituitary tumors, lymphoma, metastatic carcinoma, extension of nasal neoplasms, and histiocytic sarcoma.13 The majority of primary and secondary intracranial tumors occur in older adult dogs with the majority over 5 years of age.11, 12, 13, 14, 15, 16 Median age for dogs with meningiomas, gliomas, and choroid plexus tumors is reported as 10–11 years, 8 years, and 5–6 years, respectively.9, 12, 15, 16 Primary tumors (particularly gliomas) occasionally may be seen in younger dogs.9, 17 No sex predisposition has been reported; however it has been suggested that brain tumors generally are overrepresented in larger breeds and meningiomas are overrepresented in Golden Retrievers, Boxers, and Miniature Schnauzers. Astrocytiomas and oligodendrogliomas are highly overrepresented in specific brachycephalic breeds (Boxers, Boston Terriers, and Bulldogs), and choroid plexus tumors are overrepresented in Golden Retrievers.7, 9, 12, 15, 16, 18 Intracranial neoplasia generally is accepted to be less common in cats, although a necropsy study of approximately 4,000 cats suggested a frequency of just under 2%.19 The majority of intracranial tumors in cats are primary, with meningiomas being the predominant type. Lymphoma and pituitary tumors are the most common secondary tumors with other primary and secondary tumors, such as gliomas, occurring at relatively low frequencies compared to dogs.19, 20, 21
Advances in the treatment of intracranial tumors in dogs to date have largely been because of improvements in diagnosis, and optimization of standard therapeutic modalities such as surgical cytoreduction, radiation therapy and, to a lesser extent, chemotherapy. The marked breed association of specific tumors such as gliomas with brachycephalic breeds7, 9, 12, 18 may provide an opportunity to decrease incidence by selective breeding once provisionally defined genetic associations22, 23 are further characterized. Most of the recent major advances in human oncology have been made by elimination of environmental factors such as smoking, improved screening, and use of targeted therapies in cancers such as chronic myelogenous leukemia and breast cancer.24, 25 For neurooncology specifically, only 2 new drugs have been approved by the FDA for treatment of high‐grade gliomas in humans in the last 30 years, the chemotherapeutic temozolamide,1 which increases overall median survival in humans with grade IV astrocytomas/glioblastoma multiforme by approximately 12 weeks,26 and bevacizumab,2 which was given fast track approval, but has recently been shown to have limited if any survival benefit in a large prospective phase III clinical trial.27 It is hoped that translational studies in dogs with intracranial tumors may improve this situation for both species.
Diagnosis of Intracranial Tumors
Diagnosis of intracranial disease involves practical and economic considerations that are somewhat unique to the anatomic location. Advanced imaging such as magnetic resonance imaging (MRI) or computed tomography (CT) often is required for tentative diagnosis, and acquisition of diseased tissue for definitive histopathologic diagnosis requires either specialized biopsy equipment or invasive surgical procedures. Cerebrospinal fluid (CSF) analysis is rarely diagnostic, but its value often is overlooked. Monitoring lesions to determine biological behavior (with or without medical treatment) may provide valuable information, particularly when invasive or expensive therapeutic options are contemplated. This approach, however, may be practically limited by the expense of repeated advanced imaging techniques. The 2 major consequences of these issues are (1) much effort has been expended trying to define biomarkers of disease to aid in diagnosis, often involving advanced imaging and CSF analysis; and (2) a large amount of published information relating to treatment has been based on presumptive diagnoses, and is of limited value because many nonneoplastic lesions may have been included in therapeutic outcome data.
Histopathologic Diagnosis
Definitive diagnosis of intracranial tumors is based on histopathologic assessment using the World Health Organization (WHO) classification system. This is a continuously evolving system in human medicine with amendment of classification and tumor grade based on analysis of clinical outcomes and survival relative to specific pathologic criteria. Because there is little information relating to the natural biology of intracranial tumors in dogs, or their response to treatment, veterinary classification systems largely have been based on their tumor counterparts in humans. The only veterinary WHO classification was published in 1999,28 based on the 1993 WHO classification for humans. Since that time the system in humans has been revised 3 times and, although there are limitations, it generally is accepted that intracranial tumors in dogs should be classified using the current WHO classification system used in humans29 until specific data relating to biologic behavior are available in dogs. Molecular genetic analysis of tumors has become commonplace in human neurooncology and grading and prognostication is becoming a composite of both histopathologic and molecular criteria (discussed below).29, 30
CSF and Blood Biomarkers
As a generalization, CSF usually is not diagnostic for a specific neoplastic condition. Neoplastic cells anecdotally may be seen in CSF with almost any tumor type, but presence of neoplastic cells may occur more commonly with specific tumors such as choroid plexus tumors,16, 31 lymphoma,12, 32, 33 glioma,33 and histiocytic sarcoma.34, 35 Based on larger case series, moderate increases in total nucleated cell counts (TNCC; 5–50 cells/μL, predominantly mononuclear cells) and total protein (TP) are typical with most intracranial neoplasia, although some tumors may have normal CSF findings, and some may result in marked increases in TNCC and TP.12, 16, 33, 36, 37, 38, 39, 40 Common findings have been reported in some tumor types. For example, increased TP is seen in most choroid plexus tumors,16, 37, 40 and this may be more pronounced in choroid plexus carcinomas compared to papillomas.16 Most meningiomas have TNCC < 5 cells/μL, but increased cell counts, often with a neutrophilic component may be associated with meningiomas arising in the caudal cranial fossa.39
Defining biomarkers (in CSF or blood) for assessment of tumor burden and therapeutic response is a priority in human neurooncology.41 Classes of tumor markers include circulating tumor DNA or microRNA (miRNA) and circulating proteins such as glial fibrillary acidic protein, vascular endothelial growth factor (VEGF), matrix metalloproteinase‐9 (MMP‐9), and miRNA‐21, but ideal markers have yet to be defined.41, 42 A limited number of biomarkers (beyond tumor cells specifically) have been evaluated in CSF from dogs with intracranial tumors, including MMP‐2 and ‐9,43, 44 uric acid,45 and fibrinolytic activity (D‐dimers),46 as well as VEGF in plasma, but findings to date are similar to those in humans with regard to limitations of sensitivity and specificity.
Imaging
Although many investigators have attempted to utilize a variety of imaging techniques to diagnose, and even grade, intracranial lesions in dogs, specificity, sensitivity, or both have been shown to be consistently suboptimal in numerous studies,12, 15, 16, 36, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 particularly when applied to clinically relevant prospective random populations of patients. A majority of intracranial tumors in both dogs and cats are hypo‐ to isointense on T1‐weighted imaging, and hyperintense on T2‐weighted imaging. The majority are also contrast enhancing after administration of gadolinium‐based contrast agents.12, 15, 16, 21, 54, 55 A variety of tumor “specific” findings relating to MRI have been reported variably in several studies, some of which are listed below. Peritumoral hyperintensity (edema) on T2‐weighted images is a relatively common finding and has been suggested to be more common in astrocytomas as compared to oligodendrogliomas and in rostrotentorial as compared to infratentorial meningiomas.15, 52 Edema also has been reported to be particularly severe in relatively rare intracranial granular cell tumors.57 Contrast enhancement is generally more common in high‐grade gliomas compared to lower grade tumors, consistent with the microvascular proliferation inherent to high grade tumors.49, 52 The imaging characteristics of the most commonly occurring extra‐axial tumor, meningioma, can be indistinguishable from those of other tumors such as histiocytic sarcoma, lymphoma, and granular cell tumors.15, 57, 58 Granular cell tumors are reported to commonly have hyperintensity on precontrast T1‐weighted images, but these findings also may be present in approximately 20% of meningiomas. Large cystic structures may be associated with many tumor types, but they occur most commonly with meningiomas where their frequency is approximately 25%.15 Presence of “dural tails” and ring enhancement patterns on postcontrast T1‐weighted images has been associated with meningiomas and gliomas, respectively, but studies suggest that these patterns may be seen in a wide variety of intracranial diseases.53, 56, 59 Realistically, many therapeutic decisions are made based on presumptive imaging‐based data, but the limitations of these data and the consequences in individual animals should not be overlooked.
Future advances in the use of imaging in brain tumors of dogs likely will be based on treatment planning and assessment of therapeutic response, as definitive biopsy‐based diagnoses become more commonplace. The use of metabolic and physiologic imaging techniques has become routine in human neurooncology to define parameters such as tumor cellularity, hypoxia, vascular density, and permeability.60
Diffusion‐weighted imaging (DWI), which evaluates decreased movement of water molecules, allows for assessment of changes in tissue characteristics resulting from a variety of diseases. Although definitive diagnoses are not possible in humans61 or dogs,62 DWI may aid in the differentiation of neoplasia from conditions such as bacterial abscessation or infarction, in which diffusion is typically severely restricted (Fig 1).63 DWI has been shown to be a sensitive technique to define injury to normal canine brain following irradiation,64 and may help to differentiate tumor recurrence from the effects of treatment. In addition, diffusion tensor imaging (which measures diffusion in specific directions) may be used to define white matter tracts critical for surgical and radiation treatment planning.65
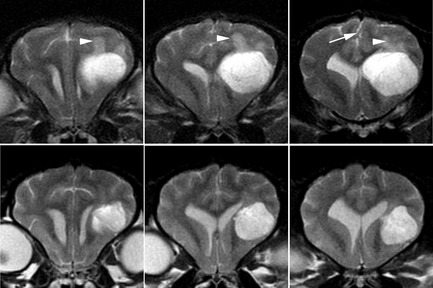
Figure 1.
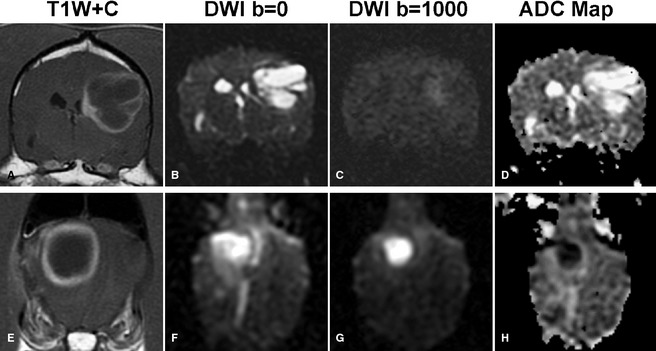
Diffusion‐weighted imaging (DWI) may aid in better defining mass lesions. (A, E) T1‐weighted postcontrast images of 2 ring‐enhancing cerebral lesions. Marked restricted diffusion, typical of abscess (E–H), but not glioblastoma multiforme (A–D) is seen as bright signal on DWI images (C, G). Apparent diffusion coefficient maps (D, H) allow background hyperintense signal because of T2 relaxation (“T2 shine through” B, F) to be differentiated from true restricted diffusion. Restricted diffusion appears hypointense on apparent diffusion coefficient (ADC) maps (D, H).
Assessment of the vascular properties of tumors may be valuable for surgical planning and also for determining response to treatment, particularly with the recent availability of antiangiogenic therapies such as bevacizumab and the multitargeted tyrosine kinase inhibitor toceranib.3 , 60 Both MRI‐ and CT‐based dynamic contrast‐enhanced techniques have been described in canine tumors to assess blood volume, perfusion, and permeability (Fig 2).66, 67 As with other imaging techniques, assessment of vascular parameters in veterinary patients has limitations for diagnosis66, 67; however, utility in monitoring vascular response to therapies including radiation has been reported.68 Anatomic definition of vascular structures associated with intracranial tumors either by magnetic resonance angiography (MRA) or contrast‐enhanced CT provides information to improve both surgical planning and to allow interventional techniques such as tumor embolization, local delivery of therapeutic agents, or both (Fig 2).69 Chemoembolization of an intracranially extended nasal tumor has been reported in a cat,70 and treatment of nasal tumors is in progress in dogs, but the more challenging goal of brain tumor embolization has not been reported in dogs. Normal canine intracranial vasculature has been defined using both contrast‐enhanced and time‐of‐flight (TOF) MRA71, 72, 73 and feasibility of imaging tumor vascularity has been demonstrated.73
Figure 2.
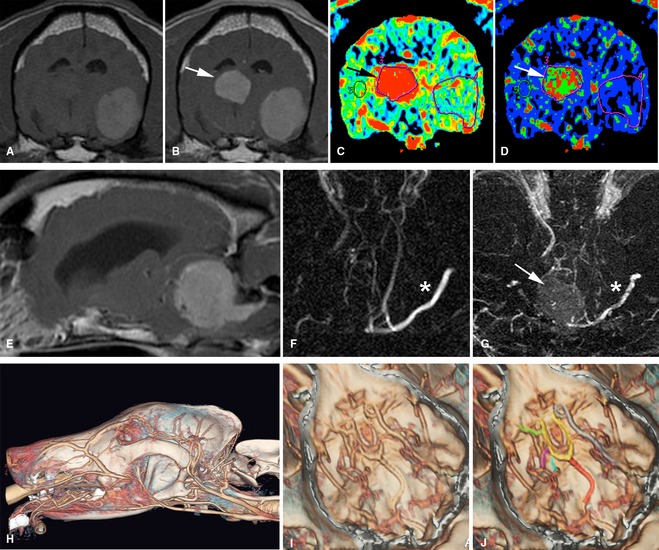
A variety of vascular imaging techniques may aid in the definition of vascular supply to, and management of, intracranial tumors. (A, B) Transverse T1‐weighted pre‐ (A) and post‐ (B) contrast magnetic resonance (MR) images of a dog with 2 tumors; a metastatic melanoma (hyperintence precontrast administration/image A) and a highly vascular choroid plexus tumor (contrast enhancing/image B). Dynamic contrast computed tomography (CT) imaging allows definition of higher blood volume (C) and perfusion (D) in the choroid plexus tumor (arrows). (E) Sagittal T1‐weighted postcontrast MRI of a cerebello‐medullary meningioma. Surgical access involves transection of the transverse sinus. Time‐of‐flight (F) and phase contrast (G) MR angiography techniques show that the tumor (arrow) has already caused substantial ablation of the transverse sinus on the side of the tumor, compared to the opposite side (*), allowing for informed surgical planning. (H–J) Contrast‐enhanced CT imaging with reconstruction of intracranial vessels. Major vessels that may be associated with intracranial tumors and interventional procedures such as tumor embolization are identified along the floor of the cranial vault (I, J) (basilar artery‐red, arterial circle‐yellow, internal carotid artery‐gray, middle cerebral artery‐green, caudal cerebral artery‐magenta, rostral cerebellar artery‐blue) (C, D, H–J courtesy of R. Pollard, M. Steffey UC Davis).
Magnetic resonance metabolic imaging of brain tumors by using proton magnetic resonance spectroscopy (1H MRS) allows determination of the chemical composition of tumor tissue, and it is a commonly used complementary technique in human neurooncology.60 MRS techniques have been described in normal canine brain,72, 74 and experimentally in canine models of nonneoplastic brain disease.75, 76, 77
Positron emission tomography (PET) and single photon emission computed tomography are functional imaging techniques that allow for qualitative and quantitative measurement of tissue metabolism together with anatomic localization after image fusion with CT or MR images. The most commonly used tracer is 2‐deoxy‐2[18F]fluoro‐d‐glucose (FDG), which reflects increased glucose metabolism in brain tissue or tumors. High background activity in metabolically active brain can be a major confounding issue, and alternative tracers such as 18F‐fluoroethythyrosine also are used in humans. Major uses for the technique include defining metabolically active areas for biopsy and definition of tumor recurrence or increasing malignancy. PET imaging has been evaluated in dogs,78, 79 and specifically in those with intracranial disease, although as with spectroscopy, clinical use is not yet routine.80, 81
Development and utilization of these metabolic and physiologic imaging techniques is very much in the developmental stage in veterinary neurooncology. Similar to human medicine, it is likely that combined use of these techniques together with standard imaging will provide the optimal information for therapeutic planning, tumor stratification, and assessment of therapeutic response, particularly when used in a linear manner.60, 82, 83
Brain Biopsy
Definitive diagnosis of intracranial lesions is based on histopathologic examination, culture, and occasionally additional analysis of tissue obtained from the lesion. In cases where surgical resection is not considered the optimal approach to treatment and diagnosis, minimally invasive biopsy techniques are considered the most appropriate way to obtain diagnostic data permitting a maximally informed approach to treatment. Free‐hand, image‐guided, and endoscope‐assisted brain biopsy has been described for dogs84, 85, 86; however, stereotactic‐guided procedures have many advantages. Both CT‐ and MRI‐based stereotactic systems were developed for dogs in the 1980s in the experimental setting,87, 88 and a variety of stereotactic approaches to clinical brain biopsy have been described in dogs and cats, the majority of which have involved CT‐based systems.89, 90, 91, 92, 93, 94, 95 Only 1 commercially available MRI‐based system has been reported,96 that is likely to become a mainstay of stereotactic biopsy in the future. Both CT‐ and MRI‐based systems have inherent advantages and disadvantages. MR‐based systems allow for better resolution of parenchymal lesions, whereas CT allows for better spatial resolution and more rapid imaging (eg, for real‐time imaging of biopsy needle placement, and postbiopsy hemorrhage assessment). Combining MR and CT images by using image fusion software (Fig 3) may maximize the benefits of both modalities. Repeated imaging is often necessary if initial diagnostic procedures are completed more than a few days before biopsy, to allow appropriate targeting of evolving lesions or to identify resolving lesions where biopsy may not be indicated. Diagnostic yield is generally >90% for neoplastic lesions, but may be considerably lower with inflammatory or infectious diseases. Morbidity and mortality associated with brain biopsy vary with the equipment used, experience of the clinician, location of the lesion, and neurologic status of the patient. Early published data described morbidity and mortality rates of 12–27% and 7–9%, respectively.89, 91 At the author's institution, complications generally occur currently in less than 5% of cases with experienced operators, and mortality associated with the procedure is rare. Although there is substantial expense associated with the procedure, increased utilization hopefully will result in more informed therapeutic planning based on accurate diagnoses, as well as providing the potential to monitor therapeutic response and biomarkers after specific therapeutic interventions.
Figure 3.
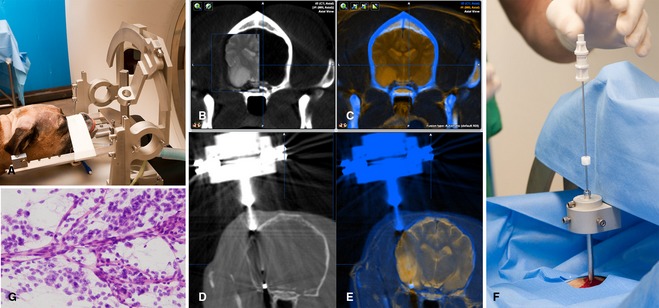
Stereotactic computed tomography (CT)‐guided brain biopsy: (A) Dog head fixed in stereotactic frame. A center of arc system allows for numerous possible biopsy trajectories. (B, C) Fusion of CT and magnetic resonance images maximizes both resolution of the lesion and spatial resolution. (D, E) Rapid CT imaging allows for real‐time imaging of biopsy needle position before biopsy. The biopsy port (approximately 8 mm length) can be seen within and at the edge of the tumor (E). (F) Insertion of biopsy needle; needle depth is determined and fixed by an adjustable collar. (G) Intraoperative rapid biopsy smears are ideally done to confirm collection of pathologic material, in this case an oligodendroglioma, before postbiopsy hemorrhage evaluation imaging and recovery.
Molecular Diagnostics
Molecular genetic characterization of neoplasia is becoming a mainstay of human neuropathology and neurooncology for both tumor classification and prognostic evaluation as well as for the appropriate application of molecular‐targeted therapies. Characterization allows for:
Defining specific subgroups of tumors that are either within histologic subtypes, or across histologic grades, relative to therapeutic outcome and prognosis.
Defining specific molecular pathways for which targeted therapeutics may be indicated to either restore or inhibit aberrant pathways, and
Defining tumor cell‐specific markers allowing tumor‐specific targeting, typically of suicide gene, or toxic therapies.
There are many examples for which molecular classification has redefined or extended previous histopathologic grading systems. Meningiomas in humans have been shown to have a molecular phenotype that predicts proliferative behavior more specifically than classical histologic subtyping,97 and extensive genomic analysis of human high‐grade astrocytomas has defined key commonly disrupted signaling pathways related to receptor tyrosine kinase (RTK)/Ras/PI3K, p53, and Rb signaling.98, 99 Similarly, the cancer genome atlas and other studies have used genome‐wide expression, copy number, epigenetic, and proteomic profiling to define 3 major molecular subclasses (proneural, mesenchymal, classical) that may form the basis for future therapeutic and prognostic stratification.98, 100, 101, 102 Despite these classifications, optimal targeted treatment still may require individualized characterization of specific markers in individual tumors (Fig 4).103, 104, 105, 106
Figure 4.
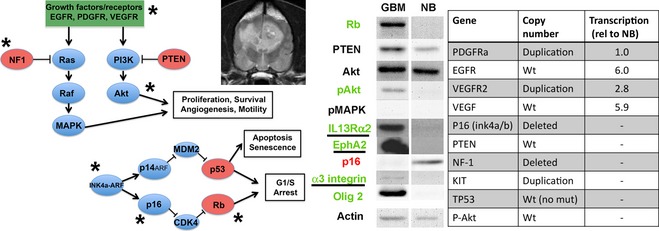
Molecular genetic characterization. Common oncogenic pathways in human glioma are shown on the left. The potential for individualized treatment targeted at specific molecular abnormalities is demonstrated in a single canine glioblastoma multiforme tumor. Western blot defines increased (green) or decreased (red) expression of key pathway proteins relative to normal brain. Chromosomal copy number alterations and transcriptionally upregulated genes for the same tumor are shown in the table on the right. Defined pathway abnormalities for this tumor are indicated by an *. Potential surface markers for targeting are underlined.
Advances in sequencing technology and availability of canine‐specific platforms have opened the door for parallel characterization of canine and human brain tumors. An ever‐expanding repertoire of targeted therapies is becoming available for cancer treatment,104 but appropriate characterization of canine tumors is critical, because although many similarities are likely, regardless of species or tumor type,107 specific differences have already been documented. Similar to human brain tumors, overexpression of cellular proliferation and apoptosis‐associated markers such as EGFR, PDGFRa, VEGFR1,2, c‐Met, IGFBP2,108, 109, 110, 111 c‐erbB2, pERK, pAkt, Bcl‐2, Bcl‐xL,111 and the angiogenic factor VEGF112, 113, 114, 115, 116 has been reported in both canine gliomas and meningiomas. Decreased progesterone receptor expression has been reported in canine meningiomas associated with higher proliferative indices and poorer prognosis,117, 118 and these features also are associated with increased VEGF expression.114
The cellular immortality‐related gene telomerase reverse transcriptase has been shown to be expressed in a variety of canine and feline brain tumors, and similar to human tumors has been associated with tumor grade.119, 120, 121 Similarly, overexpression of MMP‐2 and ‐9, E‐cadherin, claudin‐1, IL‐6, and cyclooxygenase‐2 has been documented in canine and feline meningiomas,121, 122, 123, 124, 125, 126 and MMP‐2/9 has been identified in canine gliomas and choroid plexus tumors.125 Overexpression of surface markers previously defined in human tumors, suitable for tumor targeting strategies, has been demonstrated for IL‐13RA2, EphA2, and alpha3‐beta1 integrin in a variety of canine brain tumors (Fig 4).127, 128, 129, 130 To date, global expression studies have utilized relatively limited microarray platforms and have shown some similarities to differentially expressed genes found in human tumors.131 At the epigenetic level, preliminary studies profiling genome‐wide methylation status of canine glioma suggest that hypermethylation patterns in developmentally regulated genes may be similar to those in human gliomas.132
At the chromosomal level, there are limited data defining copy number variations in canine brain tumors, and reported resolution to date has been approximately 1–3 Mb. As might be expected based on data in humans, there is decreased genomic instability in canine meningiomas compared to generally more aggressive gliomas. Potential similarities to human tumors have been documented including loss of canine chromosome (CFA) 17 and 27 (CFA 17, 27), syntenic to human chromosome (HSA) 1p and 12p; HSA 1, 12 in meningiomas.133, 134
Key hallmarks of human gliomas and meningiomas such as loss of HSA 22 (NF2 gene) in meningiomas and HSA 1p/19q in gliomas, however, have not yet been found, nor have classical glioma gene mutations such as those involving IDH1 and TP53.133, 134, 135, 136, 137 More detailed analysis of canine tumors using whole genome sequencing and single nucleotide polymorphism arrays is likely to identify additional similarities and likely novel findings, and it is probable that common key pathways in human and canine tumors will be affected by different modifications.107 Preliminary data suggest that some classical deletions in human gliomas including the INK4a/b locus and the NF‐1 gene may be present in smaller deletions,132 but, documented differences to date highlight the necessity for preclinical characterization of canine tumors before use of specific targeted approaches.
Overall, ongoing identification of aberrant gene expression as described above is likely to become increasingly important for appropriate targeting of novel therapeutic strategies. Prognostic value and therefore relevance to tumor grading in canine tumors is limited by a lack of information relating to long‐term outcome for untreated and treated tumors matched to archived tumor tissue, and establishment of these databases should be a priority for the field.
Treatment
Conclusions from most veterinary neurooncology therapeutic studies are generally limited by small case numbers, retrospective study design, and most critically, a lack of specific histologic evaluation including tumor typing and grading. The latter issues become especially critical when small case numbers are involved. Lack of easily monitored objective criteria for therapeutic response is an additional problem because repeated advanced imaging is often cost prohibitive, and many animals present with clinical signs such as seizures and clinical response may be more reflective of adjunctive antiepileptic treatment than specific tumor response. This is confounded by highly variable criteria for time of presentation and time of euthanasia. Large‐scale, multicenter therapeutic trials will be difficult to complete, and it is likely that a large amount of data addressing more focused questions in the future will be obtained from translational clinical trials that are becoming more common as the use of canine intracranial tumor models becomes more widespread. Continued collection of data relating to the natural biology and clinical course of specific tumor types and grades is critical for assessment of therapies, as is insistence on a minimum of histologic diagnosis for publication. Advances in diagnostic classification described above are likely to become more critical as molecular based therapies are developed.106
Palliative Care
Corticosteroids targeting peritumoral edema together with antiepileptic drugs to control seizures (one of the most common presenting sign for intracranial tumors)11, 12, 13, 138 form the mainstay of palliative care for intracranial neoplasia. Response to corticosteroids often can be predicted based on the degree of suspected peritumoral vasogenic edema as defined by white matter associated hyperintensity on T2‐weighted or fluid attenuated inversion recovery (FLAIR) MR images (Fig 5). When secondary obstruction of CSF drainage occurs, intraventricular shunting may provide resolution of clinical signs temporarily as a palliative measure, or as with corticosteroids, to provide time and decreased morbidity for biopsy or more definitive treatment (Fig 6). Meaningful statements regarding the natural biology of canine intracranial tumors and survival after palliative care are difficult to make based on available data for the reasons described above. There are no meaningful data relating to specific tumor types or grades. Published data suggest that for all masses combined, median survival is between approximately 1 and 10 weeks,139, 140, 141, 142 with supratentorial tumors having a better prognosis (median survival approximately 25 weeks).143 However, anecdotally many clinicians recognize that survival times for some individual patients, even those with intraaxial tumors, can be considerably longer.
Figure 5.
Palliative treatment with corticosteroids. T2‐weighted transverse images documenting the therapeutic potential of corticosteroids in a biopsy and necropsy‐confirmed anaplastic oligodendroglioma (grade III): Upper row = pretreatment, bottom row, 4 weeks after 0.5 mg/kg prednisone q24h. Even with 4 weeks of potential tumor growth, there is an overall decrease in tumor mass, with decreased presumed peritumoral vasogenic edema in surrounding white matter (arrow heads), and decreased mass effect, initially visualized as deviation of the falx cerebri (arrow).
Figure 6.
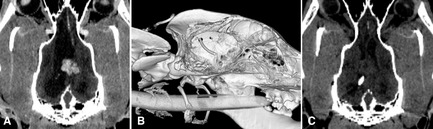
Intraventricular shunt placement provides palliative treatment of hydrocephalus secondary to obstruction of drainage from the lateral ventricles in a dog with a choroid plexus papilloma. (A) Dorsal plane reconstructed computed tomography (CT) image showing the contrast‐enhanced mass and enlarged lateral ventricle. (B) 3D‐reconstructed CT image showing the shunt tip passing intracranially through a small burr hole in the skull. (C) Postoperatively, the hyperattenuating catheter tip is seen in the now nondistended ventricle. Ventricular shunting was done to alleviate clinical signs while stereotactic radiosurgery of the tumor was undertaken.
Surgical Treatment
More than most other therapeutic modalities, efficacy of surgical cytoreduction of intracranial tumors is highly operator and equipment dependent. Most published information with meaningful case numbers is related to more easily accessible canine and feline meningiomas, with only anecdotal data for other tumor types such as gliomas and choroid plexus tumors. Currently available studies highlight the large variation in outcome, and the potential impact of applied technology. In dogs with confirmed meningiomas, standard surgical cytoreduction alone generally has been reported to result in median survival times of approximately 4.5–7 months,144, 145, 146 with an improvement in median survival to 16.5–30 months with adjunctive radiation therapy.118, 145 However, use of cortical resection, ultrasonic aspiration, or endoscope‐assisted techniques has been reported to result in median survival times of 16, 41, and 70 months, respectively, for rostrotentorial meningiomas.146, 147, 148 Given the large variation in outcome for different surgical techniques in individual studies, it is difficult to make general recommendations for surgical treatment other than the observations that cytoreduction (particularly for rostrotentorial tumors) may be “curative” for many older animals with some surgeons and techniques and that adjunctive radiation therapy has an apparent beneficial effect (Fig 7).
Figure 7.
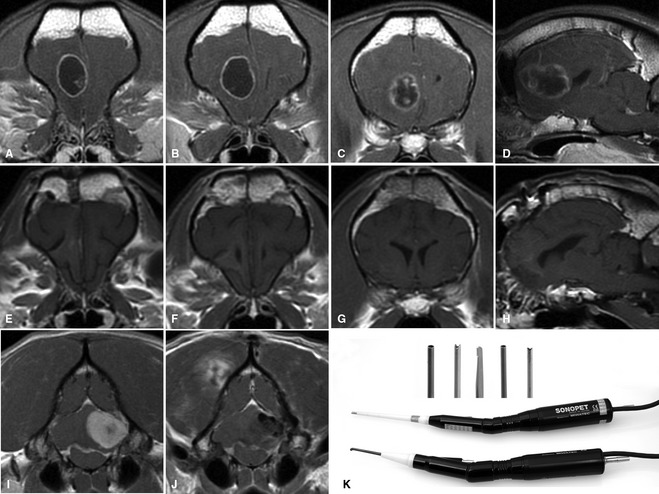
Advances in imaging and availability of neurosurgical equipment such as intraoperative stereotaxy, endoscopy, and ultrasonic aspiration has made extensive surgical resection of both extra‐axial and intraaxial tumors more commonplace. Presurgery transverse T1‐weighted postcontrast images of an intraaxial glioblastoma multiforme (A–C), sagittal (D). Postsurgery transverse (E–G), sagittal (H) TI‐weighted postcontrast images. Presurgery T1‐weighted postcontrast images of a caudal fossa extra‐axial grade II meningioma pre‐ (I) and post‐ (J) surgical resection. Gross total resection of tumor has been achieved in both cases. (K) Ultrasonic aspirators utilizing a variety of soft tissue and bone tips (inset) allow for safer and more complete resection of tumors (images courtesy of B Sturges UC Davis).
Meningiomas are the most common primary intracranial tumor in cats. Median survival time for cats with surgical cytoreduction of meningiomas is reported to be between 23 and 28 months.149, 150, 151 Feline meningiomas are generally less aggressive and locally invasive biologically than their canine counterparts, making gross total resection more likely. Information for other treatment modalities is anecdotal for both meningiomas and other sporadic tumor types in cats.
No meaningful conclusions can be made from published data relating to surgery for intraaxial tumors in dogs other than that anecdotally it can be beneficial with some animals surviving many months to over a year with surgery with or without adjunctive treatments.140, 152, 153, 154 Microsurgical transsphenoidal hypophysectomy has become a successful neurosurgical technique for the management of intracranial pituitary tumors with survival times similar to those obtained with medical management, although tumor size is a limiting factor,155 and survival times are likely to improve with application of neuronavigational devices and operator experience. Most pituitary macrotumors are best managed by radiation‐based protocols.156, 157 Application of stereotactic radiotherapy protocols, potentially using 1–3 applied doses may provide similar outcomes to hypophysectomy and are currently under investigation.
Intraoperative neuronavigation techniques using stereotactic coordinates based on either CT or MR images are standard practice in human neurooncology. Availability of veterinary MR‐based stereotactic equipment4 and custom‐made devices is likely to advance surgical treatment, particularly of intraaxial tumors, substantially in the near future (Fig 8). Several experimental and translational surgical procedures have been described in both experimental and clinical canine patients, including use of lasers,158, 159, 160 automated tissue excision systems,93 irreversible electroporation (Fig 9),161, 162 ultrasound hyperthermia,163 and robotic neurosurgery.164 Some of these techniques have shown promise and may progress to mainstream treatment. Combined with recent advances in imaging of both tumors and their vascular supply (above), as well as the potential use of fluorescent markers to aid in intraoperative tumor identification,165 continued advances in outcome are likely with surgical cytoreduction of canine and feline intracranial tumors.
Figure 8.
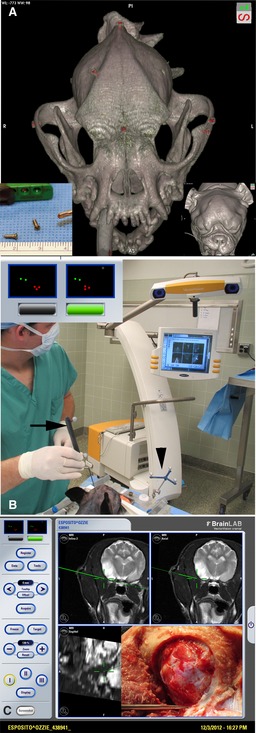
Stereotactic neuronavigation allows the surgeon to identify tumor boundaries in real‐time based on magnetic resonance (MR) or computed tomography (CT) images. This approach is particularly valuable for intraaxial tumors such as gliomas that must be approached through normal brain parenchyma. (A) 3D‐CT image showing position of titanium screws (red) used as fiducials for stereotactic referencing. (B) BrainLab VectorVision system.10 Fiducial screws are referenced on the MR images, and then in the surgery suite to the surgical pointer (arrow/green dots) and markers on the head frame (arrowhead/red dots). (C) In real‐time, the pointer is placed at the ventral edge of the craniectomy and the green line (representing the pointer) shows that the exposure is adequate to reach the ventral tumor margin.
Figure 9.
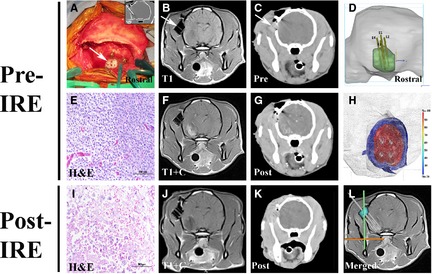
Stereotactic ablation of an anaplastic oligodendroglioma in a Boston Terrier with irreversible electroporation (IRE). (A) A threaded nylon 6‐6 probe guide pedestal (PGP‐ arrows in A–C) is implanted into the skull using titanium, self‐tapping screws, and dental acrylic to facilitate tumor biopsy (A, inset and E) and IRE electrode insertion. Pretreatment MR (B and F) and CT (C and G) images are used to plan the electrode approach trajectories and pulse delivery parameters using imaging‐based tissue segmentation (D), volumetric meshing with thermal and electrical field (H; in V/cm) threshold distributions, and finite element modeling. Posttreatment tumor biopsy and diagnostic imaging demonstrating target ablation as indicated by the necrotic tumor phenotype (I) and decrease in the contrast‐enhancing tumor burden (J, K). L‐Fused pretreatment MRI and intraoperative CT of electrode insertion into the target, with the PGP highlighted in blue (images courtesy of J Rossmeisl University of Virginia).
Chemotherapy
There is little meaningful information available relating to the efficacy of chemotherapeutic agents for canine intracranial neoplasia. Most data relate to the use of nitrosurea‐based alkylating agents such as lomustine and carmustine, or the ribunucleotide reductase inhibitor hydroxyurea. Almost all studies lack histologic diagnoses for most cases, and thus have limited value. A large retrospective study suggested no benefit for CT‐defined brain masses from lomustine (CCNU) chemotherapy compared to palliative care (93 days versus 60 days), but none of the 71 animals had a histologic diagnosis.142 Anecdotal histologically confirmed cases from published data show apparent survival benefits and occasional responses with survival of many months in some cases, but overall, chemotherapy alone appears to have limited value for intracranial tumors.147, 154, 166, 167, 168, 169 Temozolamide, a novel oral alkylating agent, has become the standard‐of‐care for adjuvant and monotherapy of high‐grade gliomas and other tumors in humans, although its use not been reported in dogs with clinical brain tumors. Canine glioma cell lines appear to have responses similar to human glioma cell lines with commonly used chemotherapeutic agents such as CCNU, CPT‐11, and temozolamide,170 and it is likely that the moderate advantages of adjuvant chemotherapy seen in human patients26, 171 are likely to be present for their canine tumor counterparts. Tumor resistance against alkylating agents is well documented in human brain tumors, and has been attributed to a variety of factors including DNA repair mechanisms, prevention of drug uptake, and inactivation and elimination of agents. Multidrug resistance proteins have been described in human brain tumors and preliminary data suggest they may play a role in drug resistance in dogs, particularly in meningiomas.172 Epigenetic silencing of the DNA repair enzyme O6‐methylguanine‐DNA‐methyltransferase by promoter hypermethylation has been associated with better responses to alkylating agents and better prognosis in human patients with gliomas,173 and is becoming a standard biomarker for therapeutic planning. Epigenetic alterations in canine brain tumors have been documented in a small number of cases,174 but their value has yet to be determined in clinical canine patients. The value of additional novel chemotherapeutic agents, either alone or in combination, combined with other therapeutic modalities or delivered in a more targeted manner remains to be evaluated. Local delivery of drug‐impregnated wafers (eg, carmustine wafers) into resection cavities has shown some small benefits in human patients with gliomas,175 but has not been evaluated in canine patients. Intratumoral delivery of a liposomal formulation of CPT‐11 has been shown to have efficacy in selected canine glioma cases, with some survival times approaching 2 years for monotherapy (Fig 10).176
Figure 10.
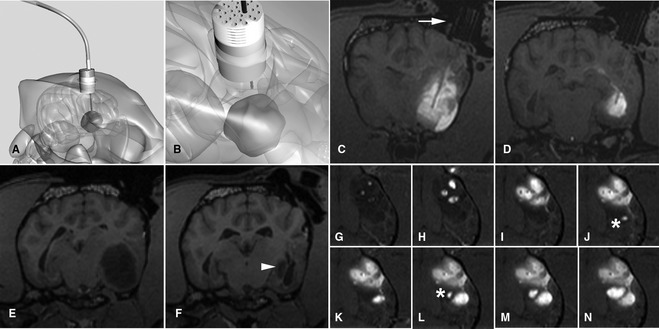
Convection‐enhanced delivery of liposomal CPT‐11 (a topoisomerase inhibitor) intratumorally by using real‐time MR imaging to optimize delivery. (A, B) Schematic representation of fused silica cannulae being guided into the tumor based on stereotactically placed guide pedestals. (C, D) Transverse T1‐weighted images at different levels showing infusate (white) of liposomal CPT‐11 and gadoteridol contrast agent within the tumor. Different cannulae can be seen highlighted against the infusate after passing down the guide pedestal (arrow). (E, F) Tumor volume (hypointense) pre‐ and posttreatment is decreased by 90% (arrowhead) after CPT‐11 infusion. (G–N) Time‐lapse imaging over approximately 2 hours infusion. Three initial cannulae result in partial tumor coverage. Real‐time imaging allows monitoring of infusion, and placement of additional cannulae (*) resulting in optimal volume of coverage.
Radiation Therapy
Radiation therapy has become a mainstay of treatment for intracranial neoplasia in both human and veterinary patients, either as a primary or adjunctive treatment. Interpretation of published data can be problematic when considering specific tumor types because of a lack of histologic diagnoses for many masses treated, and variability in radiation type and dosing schedule. Radiation therapy has been reported specifically to be beneficial when compared to surgical resection alone for treatment of meningiomas.145 In general, reported median survival times for radiation treatment alone for all masses, inraaxial masses, and extra‐axial masses are approximately 33–99 weeks, approximately 40 weeks, approximately 40–49 weeks, respectively.140, 141, 145, 152, 153, 177, 178, 179, 180 Although specific data are limited in most studies, surgery combined with radiation is reported to have improved outcomes compared to radiation alone.145, 152 Extra‐axial masses (presumptive meningioma) tend to have a better prognosis than intraaxial masses.140, 152 Standard megavoltage external beam radiation therapy has been extended by use of intensity‐modulated radiation therapy in which use of multileaf collimators that move during treatment allow for more precise, conformal delivery of radiation. Dose to tumor is increased with minimization of dose to adjacent normal structures, although extensive “inverse planning” defining tumor and normal tissues on the basis of individual CT slices is necessary. Local delivery of radiation therapy (ie, brachytherapy) has been investigated as a potential modality in experimental dogs,93, 181, 182 but overall success in humans with a variety of intracranial tumors has been limited.183, 184
More recent advances in veterinary radiation oncology for brain tumors have been driven by the availability of stereotactic radiotherapy (SRT) equipment and procedures, in which radiation is delivered to sterotactically defined tumor volumes. Radiation may be delivered by multiple cobalt sources,5 or linear accelerators delivering extremely precise, high dose/gradient plans. These may involve multiple static beams using a 6 MV linear accelerator on a robotic arm with 6 degrees of freedom,6 or standard linear accelerators fitted with stereotactic cones or micromultileaf collimators using multiple arcs planned around isocenters. SRT uses image‐guided (MRI or CT), forward‐based planning, and requires stringent quality assurance (Fig 11). SRT has become widely used for the treatment of nonresectable intracranial masses, either intraaxial or involving the skull base. Experience with skull base meningiomas and gliomas in humans suggests responses may approach those seen with surgically resected tumors.185, 186 One of the major advantages of SRT techniques is the ability to deliver the therapeutic dose of radiation in 2–5 fractions (single dose treatments are referred to as stereotactic radiosurgery [SRS]) compared to 16–20 fractions for standard radiation protocols. This is particularly relevant in veterinary patients, where general anesthesia is required for each treatment. There are limitations to the size of masses that can be treated with SRT (several cms), and it is generally not suitable for treating microscopic residual disease (eg, after surgical resection). Availability of treatment centers is limited, and only linear accelerator‐based systems have been used in dogs and cats, but preliminary data suggest that efficacy is comparable to standard radiation protocols with potentially fewer short‐term adverse effects.187, 188, 189, 190, 191 Final evaluation of SRT will be dependent on long‐term assessment of histologically confirmed cases. Boron neutron capture treatment is a localized radiation therapy depending on preferential local delivery of 10B to tumor tissue followed by delivery of thermal neutrons. Resultant 7Li nuclei and 4He (α‐particles) produce high‐dose radiotherapy with potential for selective killing of 10B‐loaded cells. Preliminary translational studies in dogs with a variety of intracranial tumors demonstrated the feasibility of the approach and anecdotal therapeutic successes with and without surgical cytoreduction.192, 193 Although availability of equipment is limiting, recent advances in more tumor‐selective boron delivery drugs may improve the limited clinical efficacy and toxicity (eg, radiation necrosis) in the future.194
Figure 11.
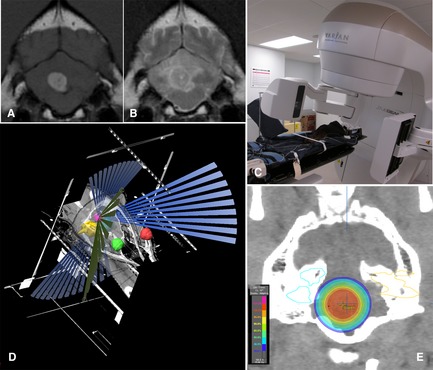
(A, B) T1‐weighted postcontrast and T2‐weighted transverse images of a 4th ventricle choroid plexus tumor. (C) Dog positioned in stereotactic thermoplastic head restraint. The VARIAN trueBEAM linear accelerator11 is equipped with a 2.5‐mm leaf multileaf collimator, a couch with 0.1‐mm incremental movement, and on‐board Kv, MV and cone beam CT to allow precise stereotactic delivery of radiation. (D) BrainLab planning system10 showing the planned treatment trajectories to the tumor (magenta) sparing defined vital structures (eyes‐red/green, inner ears‐yellow/blue). (E) Transverse CT image with isodose planning superimposed (images courtesy of M. Kent, UC Davis).
Novel Therapies
Recognition of small animal diseases as clinically relevant translational models for human disease has opened up numerous collaborative opportunities for veterinarians, most notably in the field of oncology. Canine brain tumor patients are increasingly being enrolled in a variety of clinical trials. Ongoing areas of research involving veterinary centers can be broadly divided into:
Novel delivery approaches to circumvent drug delivery limitations because of the blood–brain barrier;
therapies targeting aberrant molecular pathways;
toxin or suicide gene therapies targeted to tumor cell‐specific markers; and
immunotherapies.
Targeting of brain tumors at the gross level has been advanced by techniques such as convection‐enhanced delivery (CED), in which infusion of therapeutic agents directly into tumor tissue results in the potential for extremely high intratumoral drug concentrations with minimal to no systemic toxicity. The technique involves delivery of macromolecules by bulk flow using low pressures and specifically designed catheters, and allows clinically relevant volumes of therapeutic agents to be delivered, usually over several hours to days.195 Recent advances in the technique have allowed for real‐time imaging of infusions allowing for both accurate planning and meaningful assessment of therapeutic outcome (Fig 10). A variety of imaging agents have been used including gadolinium, iron oxide nanoparticles, and PET tracers.176, 196, 197, 198 Specifically in canine brain tumors, CED of liposomal CPT‐11, and EGFRvIII‐antibody bioconjugated magnetic iron oxide nanoparticles have been shown to have efficacy in canine gliomas, even as monotherapies, with minimal adverse effects (Figs 10, 12).176, 199 CED approaches are often limited by an inability to distribute therapeutic agents to the entire tumor volume. Advances in catheter designs, predictive imaging software, and therapeutic agent strategies are helping to improve volumes of distribution throughout heterogenous tumors, and include the use of arborizing fiberoptic catheters and local hyperthermia.175, 200 CED infusion of replication competent retroviral vectors capable of tumor spread beyond the borders of the CED distribution is an alternative strategy to maximize tumor coverage, and has been utilized in both human and canine clinical trials to deliver suicide gene therapy vectors, although efficacy has not yet been shown.201, 202
Figure 12.
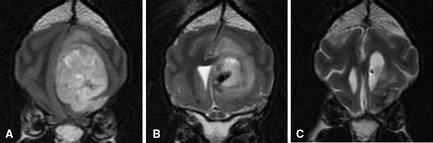
Intratumoral delivery of iron oxide nanoparticles. (A) Pretreatment T2‐weighted transverse image showing a large intraaxial oligodendroglioma. (B) Seven days after surgical resection, cetuximab12‐conjugated iron oxide nanoparticles targeting to EGFR were infused into the residual tumor. Susceptibility artifact generated by the iron particles allows the infusion to be determined as an area of hypointensity within the residual tumor mass. (C) Nanoparticles are still apparent 4 weeks postinfusion and tumor mass effect is substantially decreased. The dog is alive 2 years posttreatment (images courtesy of S. Platt University of Georgia).
Many experimental gene therapies have been developed for trials in humans utilizing a variety of viral vectors (often nonreplicating or conditionally replicative), most commonly adenovirus, adeno‐associated virus, herpes simplex virus, and retrovirus. Common therapeutic strategies include suicide gene therapy, oncolytic treatment, immunomodultion, gene replacement, proapoptotic treatment, and antiangiogenesis.175, 203 Successful delivery or efficacy of gene therapy approaches using viral vectors and plasmid DNA has been shown experimentally in canine brain tumor cells and brain using adenoviral,204, 205, 206, 207, 208, 209 retroviral201, 202, 210 herpes,211 and adeno‐associated viral212, 213, 214 delivery. Few viral therapies have progressed to phase III clinical trials, and none have been shown to have significant efficacy in high‐grade brain tumors in phase III trials to date.175
Targeting of defined aberrant pathways in oncology has resulted in some of the most dramatic improvements in survival times for several human cancers, most notably trastuzumab7 antibody targeting of Her2/Neu overexpressing breast cancers24 and small molecule inhibitor imatinib8 targeting of BCR‐ABL positive chronic myelogenous leukemia.25 A majority of targeted therapies involve either antibodies or small molecule inhibitors, and many have been investigated in human brain tumors with minimal activity demonstrated to date.104 This may be a reflection of many factors including insufficient characterization of both tumors and patients, as well as the potential need for multiple target strategies. Two small molecule inhibitors have been approved for use in veterinary medicine, toceranib phosphate3 which blocks a variety of RTKs including VEGFR2, PDGFRalpha/beta, KIT, and FLt3, and masitinib9 which inhibits PDGFRalpha/beta and KIT. Veterinary trials with toceranib and masitinib have shown benefit in several cancers but have not been reported for brain tumors. Documented overexpression of VEGF, VEGF receptors, and PDGFR alpha in some canine brain tumors108, 109, 110, 111, 112, 113, 114, 115, 116 may justify trials with these or similar small molecules in defined patients.
A growing body of evidence has implicated tumor cells with stem cell‐like properties as a potential source of both tumor initiation and tumor recurrence or resistance to treatment.215, 216, 217 Although this is still a controversial area of research, many investigators have defined populations of tumor cells that have genetic and epigenetic phenotypes similar to primitive precursor cells with molecular profiles more typical of cells during brain neuro‐ and gliogenesis and development.218 Targeting either stem cell surface markers or dysregulated “developmental” pathways in tumors is an attractive and ongoing area of research. Stem‐like cells expressing putative developmental markers such as CD133, Olig2, and nestin have been described by several authors in canine gliomas (Fig 4), and preliminary data suggest that epigenetic alterations in canine glioma may parallel the developmental profile seen in human tumors.219, 220, 221, 222, 223 An alternative utilization of stem cells in neurooncology is the exploitation of the inherent tumor‐tropic properties of normal or modified stem cells. The ability to target distant, invasive tumor cells and deliver a variety of therapeutic agents is a developing and promising field.203
Targeting of aberrantly expressed surface markers on tumor cells has been exploited as a strategy for delivering imaging or therapeutic agents specifically to tumors. Ideal markers are expressed in all tumor cells, ideally in all tumors, and have minimal to no expression in either local (eg, brain) or systemic tissues, if systemic delivery strategies are to be used. Several markers such as IL‐13 receptor alpha 2, EGFR, and transferring receptor, either alone or in combination, have been targeted in human neurooncology to deliver toxin based or suicide gene therapies.175 Similar to human gliomas, most canine gliomas overexpress both IL‐13 alpha 2 receptor and EphA2 receptor.128, 129 Trials in humans using Pseudomonas‐derived IL‐13 toxin conjugates have shown some efficacy, and trials in dogs utilizing similar canine optimized toxins127, 128, 129 are planned. Similarly, targeting of canine tumors overexpressing EGFR using antibodies conjugated to iron nanoparticles is in progress.199
Small peptides have major advantages over antibodies for tumor targeting in that they are less immunogenic, have longer tissue half‐lives, and have the potential to be readily modified and conjugated for a variety of therapeutic or imaging options. Random screening of peptide libraries is an efficient method to define tumor‐specific peptides,224 and has been done for several canine cancers including lymphoma, melanoma, and glioma.130, 225, 226 Tumor‐specific targeting of glioma using peptides recognizing alpha 3 beta 1 integrin has been demonstrated in human cancers.227 Similar findings in canine glioma as well as in vivo demonstration of the feasibility of peptide targeting in dogs228, 229 opens the possibility of future peptide‐conjugated therapies for canine glioma (Fig 13).
Figure 13.
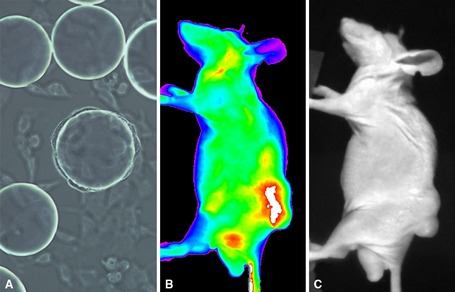
Isolation of canine glioma‐targeting peptides for delivery of imaging and therapeutic agents. Tumor‐specific peptides have many advantages over more traditional antibody based targeting strategies. (A) Canine glioma cells bind to bead‐specific peptide sequences in random screening libraries. (B, C) Isolation of bead peptides and conjugation to fluorescent markers allows targeting to canine glioma tumors grown in mouse subcutaneous models.
Immunotherapy
Augmentation of the patient's T cell‐mediated immune response against neoplastic cells, normally limited by the brain's immuno‐privileged niche, is a developing field in humans for both gliomas and on a smaller scale meningiomas.203, 230, 231, 232 Several approaches are being explored including gene therapy delivery of immunostimulatory genes such as IL‐2, 4, 12, TNF alpha, interferon alpha, beta, and gamma and dendritic cell growth factors such as Flt3L. Several “vaccine”‐based approaches have been developed including vaccination with patient dendritic cells “primed” with tumor antigen, tumor peptides, heat shock proteins, and autologous and allogenic tumor cell preparations.203, 230, 231, 232, 233 Limited information is available defining immune cell activity in canine brain tumors, but preliminary studies defining immune cell infiltration in canine meningiomas,234 and the ability of Flt3L to stimulate canine dendritic cells,235 suggest that there will be many similarities to human tumors. Translational studies in dogs with glioma using tumor cell lysate/CpG vaccines, combined with postsurgical intracavitary delivery of IFNg via an adenoviral vector, have demonstrated the feasibility of immunotherapy in dogs, with tumor‐reactive IgG and CD8+ T cells being documented in 1 reported case.206, 236 Similar studies using autologous tumor lysate vaccines combined with toll‐like receptor ligands (CpG, imiquimod) after resection of meningiomas in dogs also have been reported.237 Treatment resulted in the production of polyclonal antibody responses in all dogs, with infiltration of plasma cells into surrounding brain tissue. Additional studies investigating combinations of local adenoviral gene therapy delivery of HSV‐tk suicide genes and Flt3L dendritic growth factor postresection and tumor lysate vaccines derived under varying oxygen tensions are in progress.236 Although efficacy of immune‐based therapies has yet to be shown in phase III trials in humans or veterinary patients, preliminary results are encouraging and show the feasibility of these approaches.
Future
Veterinary neurooncology still has a large amount of benefit to be obtained from application of currently available techniques and advancements in standard surgical, chemotherapeutic, and radiation‐based therapies, but expense, species‐specific factors, and availability may be limiting in some areas.
Rapid advances in global analysis of cancer molecular phenotypes together with industrial scale development of targeted small molecule therapeutics are likely to provide the greatest opportunity for advances in outcome in both human and veterinary neurooncology. Realistically, companion animal neurooncology will rely on use or modification of therapeutics developed for human patients, and although it appears that most cancers follow similar developmental pathways, subtle differences may be critical for appropriate application. Continued in‐depth evaluation of both the molecular genetics and natural biology of companion animal intracranial tumors will be critical for optimal future outcomes for patients with these tumors.
Acknowledgment
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Biography
Peter J. Dickinson BVSc, PhD, Diplomate ACVIM (Neurology) Peter Dickinson graduated from Liverpool University Veterinary School in 1989. Following 1 year in mixed general practice he completed a 2 year surgery/anesthesia internship at Glasgow University Veterinary School. He received his PhD in developmental neuroscience in 1995, also at Glasgow University, before completing a Neurology/Neurosurgery residency at the University of California, Davis. He is currently Professor of Neurology/Neurosurgery at UC Davis Veterinary School and is a diplomate of the American College of Veterinary Medicine (Neurology). His clinical and research interest has been in the field of neurooncology and he is director of the Petersen Foundation Brain Tumor Laboratory.

Footnotes
Temodar; Merk & Co, Inc, Whitehouse Station, NJ
Avastin; Genentech Inc, South San Francisco, CA
SU11654 Palladia; Pfizer Animal Health, New York, NY
Brainsight Vet; Rogue Research Inc, Montreal, QC, Canada
Gamma Knife Elekta AB, Stockholm, Sweden
CyberKnife; Accuray, Sunnyvale, CA
Herceptin; Genentech Inc
Gleevec; Novartis Pharmaceuticals Corporation, East Hanover, NJ
Kinavet, Masivet; AB Science, Short Hills, NJ
VectorVision, BRAINLAB; Feldkirschen, Germany
trueBEAM; Varian Medical Systems, Inc, Palo Alto, CA
Erbitux; ImClone Systems Inc, Somerville, NJ
References
- 1. Kimmelman J, Nalbantoglu J. Faithful companions: A proposal for neurooncology trials in pet dogs. Cancer Res 2007;67:4541–4544. [DOI] [PubMed] [Google Scholar]
- 2. Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer 2008;8:147–156. [DOI] [PubMed] [Google Scholar]
- 3. Candolfi M, Curtin JF, Nichols WS, et al. Intracranial glioblastoma models in preclinical neuro‐oncology: Neuropathological characterization and tumor progression. J Neurooncol 2007;85:133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorn CR, Taylor DO, Frye FL, et al. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. I. Methodology and description of cases. J Natl Cancer Inst 1968;40:295–305. [PubMed] [Google Scholar]
- 5. Schneider R. General considerations In: Moulton JE, ed. Tumors in Domestic Animals, 2nd ed Berkley: University of California Press; 1978:1–15. [Google Scholar]
- 6. Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 2012;14(Suppl 5):v1–v49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGrath JT. Intracranial pathology of the dog. Acta Neuropathol (Berl) 1962;(Suppl I):3–4. [Google Scholar]
- 8. Priester WA, Mantel N. Occurrence of tumors in domestic animals. Data from 12 United States and Canadian colleges of veterinary medicine. J Natl Cancer Inst 1971;47:1333–1344. [PubMed] [Google Scholar]
- 9. Song RB, Vite CH, Bradley CW, et al. Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age, and body weight. J Vet Intern Med 2013;27:1143–1152. [DOI] [PubMed] [Google Scholar]
- 10. Klotz M. Incidence of brain tumors in patients hospitalized for chronic mental disorders. Psychiatr Q 1957;31:669–680. [DOI] [PubMed] [Google Scholar]
- 11. Bagley RS, Gavin PR, Moore MP, et al. Clinical signs associated with brain tumors in dogs: 97 cases (1992–1997). J Am Vet Med Assoc 1999;215:818–819. [PubMed] [Google Scholar]
- 12. Snyder JM, Shofer FS, Van Winkle TJ, et al. Canine intracranial primary neoplasia: 173 cases (1986–2003). J Vet Intern Med 2006;20:669–675. [DOI] [PubMed] [Google Scholar]
- 13. Snyder JM, Lipitz L, Skorupski KA, et al. Secondary intracranial neoplasia in the dog: 177 cases (1986–2003). J Vet Intern Med 2008;22:172–177. [DOI] [PubMed] [Google Scholar]
- 14. Song YK, Liu F, Chu S, et al. Characterization of cationic liposome‐mediated gene transfer in vivo by intravenous administration. Hum Gene Ther 1997;8:1585–1594. [DOI] [PubMed] [Google Scholar]
- 15. Sturges BK, Dickinson PJ, Bollen AW, et al. Magnetic resonance imaging and histological classification of intracranial meningiomas in 112 dogs. J Vet Intern Med 2008;22:586–595. [DOI] [PubMed] [Google Scholar]
- 16. Westworth DR, Dickinson PJ, Vernau W, et al. Choroid plexus tumors in 56 dogs (1985–2007). J Vet Intern Med 2008;22:1157–1165. [DOI] [PubMed] [Google Scholar]
- 17. Kube SA, Bruyette DS, Hanson SM. Astrocytomas in young dogs. J Am Anim Hosp Assoc 2003;39:288–293. [DOI] [PubMed] [Google Scholar]
- 18. Hayes HM, Priester WA Jr, Pendergrass TW. Occurrence of nervous‐tissue tumors in cattle, horses, cats and dogs. Int J Cancer 1975;15:39–47. [DOI] [PubMed] [Google Scholar]
- 19. Zaki FA, Hurvitz AI. Spontaneous neoplasms of the central nervous system of the cat. J Small Anim Pract 1976;17:773–782. [DOI] [PubMed] [Google Scholar]
- 20. Tomek A, Cizinauskas S, Doherr M, et al. Intracranial neoplasia in 61 cats: Localisation, tumour types and seizure patterns. J Feline Med Surg 2006;8:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Troxel MT, Vite CH, Massicotte C, et al. Magnetic resonance imaging features of feline intracranial neoplasia: Retrospective analysis of 46 cats. J Vet Intern Med 2004;18:176–189. [DOI] [PubMed] [Google Scholar]
- 22. Bannasch D, Young A, Myers J, et al. Localization of canine brachycephaly using an across breed mapping approach. PLoS One 2010;5:e9632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Truvé K, Dickinson P, York D, et al. Evaluation of selective sweeps for brachycephaly in dogs and associated susceptibility loci for glioma In: Proceedings of the 6th International Conference on Advances in Canine and Feline Genomics and Inherited Diseases, Visby, Sweden, May 28‐June 1, 2012. [Google Scholar]
- 24. Slamon DJ, Leyland‐Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 25. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR‐ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001;344:1031–1037. [DOI] [PubMed] [Google Scholar]
- 26. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‐year analysis of the EORTC‐NCIC trial. Lancet Oncol 2009;10:459–466. [DOI] [PubMed] [Google Scholar]
- 27. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koestner A, Bilzer T, Fatzer R, et al. Histological Classification of Tumors of the Nervous System of Domestic Animals, 2nd ed Washington, DC: The Armed Forces Institute of Pathology; 1999:71. [Google Scholar]
- 29. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl) 2007;114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumors of the Central Nervous System, 4th ed Geneva: WHO Press; 2007. [Google Scholar]
- 31. Grevel V, Machus B. Diagnosing brain tumors with a CSF sedimantation technique. Vet Med Rep 1990;2:403–408. [Google Scholar]
- 32. Palus V, Volk HA, Lamb CR, et al. MRI features of CNS lymphoma in dogs and cats. Vet Radiol Ultrasound 2012;53:44–49. [DOI] [PubMed] [Google Scholar]
- 33. Vandevelde M, Richard A, Fankhauser R. Liquoruntersuchungen bei neurologisch kranken hunden und katzen. Schweiz Arch Tierheilk 1987;129:443–456. [PubMed] [Google Scholar]
- 34. Tzipory L, Vernau KM, Sturges BK, et al. Antemortem diagnosis of localized central nervous system histiocytic sarcoma in 2 dogs. J Vet Intern Med 2009;23:369–374. [DOI] [PubMed] [Google Scholar]
- 35. Zimmerman K, Almy F, Carter L, et al. Cerebrospinal fluid from a 10‐year‐old dog with a single seizure episode. Vet Clin Pathol 2006;35:127–131. [DOI] [PubMed] [Google Scholar]
- 36. Bohn AA, Wills TB, West CL, et al. Cerebrospinal fluid analysis and magnetic resonance imaging in the diagnosis of neurologic disease in dogs: A retrospective study. Vet Clin Pathol 2006;35:315–320. [DOI] [PubMed] [Google Scholar]
- 37. Bailey CS, Higgins RJ. Characteristics of cisternal cerebrospinal fluid associated with primary brain tumors in the dog: A retrospective study. J Am Vet Med Assoc 1986;188:414–417. [PubMed] [Google Scholar]
- 38. Rand JS, Parent J, Percy D, et al. Clinical, cerebrospinal fluid, and histological data from thirty‐four cats with primary noninflammatory disease of the central nervous system. Can Vet J 1994;35:174–181. [PMC free article] [PubMed] [Google Scholar]
- 39. Dickinson PJ, Sturges BK, Kass PH, et al. Characteristics of cisternal cerebrospinal fluid associated with intracranial meningiomas in dogs: 56 cases (1985–2004). J Am Vet Med Assoc 2006;228:564–567. [DOI] [PubMed] [Google Scholar]
- 40. Moore MP, Gavin PR, Bagley RS, et al. Cerebrospinal fluid analysis in dogs with intracranial tumors In: Proceedings of the American College of Veterinary Internal Medicine Forum, San Franscisco, CA, June 2, 1994:917–921. [Google Scholar]
- 41. Holdhoff M, Yovino SG, Boadu O, et al. Blood‐based biomarkers for malignant gliomas. J Neurooncol 2013;113:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ilhan‐Mutlu A, Wagner L, Preusser M. Circulating biomarkers of CNS tumors: An update. Biomark Med 2013;7:267–285. [DOI] [PubMed] [Google Scholar]
- 43. Mariani CL, Boozer LB, Braxton AM, et al. Evaluation of matrix metalloproteinase‐2 and ‐9 in the cerebrospinal fluid of dogs with intracranial tumors. Am J Vet Res 2013;74:122–129. [DOI] [PubMed] [Google Scholar]
- 44. Turba ME, Forni M, Gandini G, et al. Recruited leukocytes and local synthesis account for increased matrix metalloproteinase‐9 activity in cerebrospinal fluid of dogs with central nervous system neoplasm. J Neurooncol 2007;81:123–129. [DOI] [PubMed] [Google Scholar]
- 45. Platt SR, Marlin D, Smith N, et al. Increased cerebrospinal fluid uric acid concentrations in dogs with intracranial meningioma. Vet Rec 2006;158:830. [DOI] [PubMed] [Google Scholar]
- 46. de la Fuente C, Monreal L, Ceron J, et al. Fibrinolytic activity in cerebrospinal fluid of dogs with different neurological disorders. J Vet Intern Med 2012;26:1365–1373. [DOI] [PubMed] [Google Scholar]
- 47. Cervera V, Mai W, Vite CH, et al. Comparative magnetic resonance imaging findings between gliomas and presumed cerebrovascular accidents in dogs. Vet Radiol Ultrasound 2011;52:33–40. [PubMed] [Google Scholar]
- 48. Leclerc MK, d'Anjou MA, Blond L, et al. Interobserver agreement and diagnostic accuracy of brain magnetic resonance imaging in dogs. J Am Vet Med Assoc 2013;242:1688–1695. [DOI] [PubMed] [Google Scholar]
- 49. Young BD, Levine JM, Porter BF, et al. Magnetic resonance imaging features of intracranial astrocytomas and oligodendrogliomas in dogs. Vet Radiol Ultrasound 2011;52:132–141. [DOI] [PubMed] [Google Scholar]
- 50. Keenihan EK, Summers BA, David FH, et al. Canine meningeal disease: Associations between magnetic resonance imaging signs and histologic findings. Vet Radiol Ultrasound 2013;54:504–515. [DOI] [PubMed] [Google Scholar]
- 51. Lamb CR, Croson PJ, Cappello R, et al. Magnetic resonance imaging findings in 25 dogs with inflammatory cerebrospinal fluid. Vet Radiol Ultrasound 2005;46:17–22. [DOI] [PubMed] [Google Scholar]
- 52. Bentley RT, Ober CP, Anderson KL, et al. Canine intracranial gliomas: Relationship between magnetic resonance imaging criteria and tumor type and grade. Vet J 2013;198:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cherubini GB, Mantis P, Martinez TA, et al. Utility of magnetic resonance imaging for distinguishing neoplastic from non‐neoplastic brain lesions in dogs and cats. Vet Radiol Ultrasound 2005;46:384–387. [DOI] [PubMed] [Google Scholar]
- 54. Kraft SL, Gavin PR, DeHaan C, et al. Retrospective review of 50 canine intracranial tumors evaluated by magnetic resonance imaging. J Vet Intern Med 1997;11:218–225. [DOI] [PubMed] [Google Scholar]
- 55. Rodenas S, Pumarola M, Gaitero L, et al. Magnetic resonance imaging findings in 40 dogs with histologically confirmed intracranial tumours. Vet J 2011;187:85–91. [DOI] [PubMed] [Google Scholar]
- 56. Singh JB, Oevermann A, Lang J, et al. Contrast media enhancement of intracranial lesions in magnetic resonance imaging does not reflect histopathologic findings consistently. Vet Radiol Ultrasound 2011;52:619–626. [DOI] [PubMed] [Google Scholar]
- 57. Anwer CC, Vernau KM, Higgins RJ, et al. Magnetic resonance imaging features of intracranial granular cell tumors in six dogs. Vet Radiol Ultrasound 2013;54:271–277. [DOI] [PubMed] [Google Scholar]
- 58. Tamura S, Tamura Y, Nakamoto Y, et al. MR imaging of histiocytic sarcoma of the canine brain. Vet Radiol Ultrasound 2009;50:178–181. [DOI] [PubMed] [Google Scholar]
- 59. Graham JP, Newell SM, Voges AK, et al. The dural tail sign in the diagnosis of meningiomas. Vet Radiol Ultrasound 1998;39:297–302. [DOI] [PubMed] [Google Scholar]
- 60. Nelson SJ. Assessment of therapeutic response and treatment planning for brain tumors using metabolic and physiological MRI. NMR Biomed 2011;24:734–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stadnik TW, Chaskis C, Michotte A, et al. Diffusion‐weighted MR imaging of intracerebral masses: Comparison with conventional MR imaging and histologic findings. AJNR Am J Neuroradiol 2001;22:969–976. [PMC free article] [PubMed] [Google Scholar]
- 62. Sutherland‐Smith J, King R, Faissler D, et al. Magnetic resonance imaging apparent diffusion coefficients for histologically confirmed intracranial lesions in dogs. Vet Radiol Ultrasound 2011;52:142–148. [DOI] [PubMed] [Google Scholar]
- 63. Desprechins B, Stadnik T, Koerts G, et al. Use of diffusion‐weighted MR imaging in differential diagnosis between intracerebral necrotic tumors and cerebral abscesses. AJNR Am J Neuroradiol 1999;20:1252–1257. [PMC free article] [PubMed] [Google Scholar]
- 64. Wang Y, Lv X, Gong H, et al. Acute irradiation injury of canine brain with pathology control is detected by diffusion‐weighted imaging of MRI. Clin Imaging 2013;37:440–445. [DOI] [PubMed] [Google Scholar]
- 65. Gerstner ER, Sorensen AG. Diffusion and diffusion tensor imaging in brain cancer. Semin Radiat Oncol 2011;21:141–146. [DOI] [PubMed] [Google Scholar]
- 66. MacLeod AG, Dickinson PJ, LeCouteur RA, et al. Quantitative assessment of blood volume and permeability in cerebral mass lesions using dynamic contrast‐enhanced computed tomography in the dog. Acad Radiol 2009;16:1187–1195. [DOI] [PubMed] [Google Scholar]
- 67. Zhao Q, Lee S, Kent M, et al. Dynamic contrast‐enhanced magnetic resonance imaging of canine brain tumors. Vet Radiol Ultrasound 2010;51:122–129. [DOI] [PubMed] [Google Scholar]
- 68. Zwingenberger AL, Pollard RE, Kent MS. Measuring response of brain tumors to stereotactic radiosurgery:Interim results. Vet Radiol Ultrasound 2010;51:577. [Google Scholar]
- 69. Duffis EJ, Gandhi CD, Prestigiacomo CJ, et al. Head, neck, and brain tumor embolization guidelines. J Neurointerv Surg 2012;4:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marioni‐Henry K, Schwarz T, Weisse C, et al. Cystic nasal adenocarcinoma in a cat treated with piroxicam and chemoembolization. J Am Anim Hosp Assoc 2007;43:347–351. [DOI] [PubMed] [Google Scholar]
- 71. Jacqmot OD, Snaps FR, Maquet NM, et al. Arterial head vascularization cartographies of normal metencephalic dogs using magnetic resonance angiography. Anat Rec (Hoboken) 2011;294:1834–1841. [DOI] [PubMed] [Google Scholar]
- 72. Martin‐Vaquero P, da Costa RC, Echandi RL, et al. Magnetic resonance spectroscopy of the canine brain at 3.0 T and 7.0 T. Res Vet Sci 2012;93:427–429. [DOI] [PubMed] [Google Scholar]
- 73. Sager M, Assheuer J, Trummler H, et al. Contrast‐enhanced magnetic resonance angiography (CE‐MRA) of intra‐ and extra‐cranial vessels in dogs. Vet J 2009;179:92–100. [DOI] [PubMed] [Google Scholar]
- 74. Shores A, Warber‐Matich S, Cooper TG. The role of magnetic resonance spectroscopy in neuro‐oncology. Semin Vet Med Surg (Small Anim) 1990;5:237–240. [PubMed] [Google Scholar]
- 75. Barreiro CJ, Williams JA, Fitton TP, et al. Noninvasive assessment of brain injury in a canine model of hypothermic circulatory arrest using magnetic resonance spectroscopy. Ann Thorac Surg 2006;81:1593–1598. [DOI] [PubMed] [Google Scholar]
- 76. Kang BT, Jang DP, Lee JH, et al. Detection of cerebral metabolites in a canine model of ischemic stroke using 1H magnetic resonance spectroscopy. Res Vet Sci 2009;87:300–306. [DOI] [PubMed] [Google Scholar]
- 77. Lee SH, Kim SY, Woo DC, et al. Differential neurochemical responses of the canine striatum with pentobarbital or ketamine anesthesia: A 3T proton MRS study. J Vet Med Sci 2010;72:583–587. [DOI] [PubMed] [Google Scholar]
- 78. Hansen AE, McEvoy F, Engelholm SA, et al. FDG PET/CT imaging in canine cancer patients. Vet Radiol Ultrasound 2011;52:201–206. [DOI] [PubMed] [Google Scholar]
- 79. Kang BT, Son YD, Lee SR, et al. FDG uptake of normal canine brain assessed by high‐resolution research tomography‐positron emission tomography and 7 T‐magnetic resonance imaging. J Vet Med Sci 2012;74:1261–1267. [DOI] [PubMed] [Google Scholar]
- 80. Kang BT, Kim SG, Lim CY, et al. Correlation between fluorodeoxyglucose positron emission tomography and magnetic resonance imaging findings of non‐suppurative meningoencephalitis in 5 dogs. Can Vet J 2010;51:986–992. [PMC free article] [PubMed] [Google Scholar]
- 81. Kang BT, Park C, Yoo JH, et al. 18F‐fluorodeoxyglucose positron emission tomography and magnetic resonance imaging findings of primary intracranial histiocytic sarcoma in a dog. J Vet Med Sci 2009;71:1397–1401. [DOI] [PubMed] [Google Scholar]
- 82. Horska A, Barker PB. Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin N Am 2010;20:293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li Y, Lupo JM, Polley MY, et al. Serial analysis of imaging parameters in patients with newly diagnosed glioblastoma multiforme. Neuro Oncol 2011;13:546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Flegel T, Oevermann A, Oechtering G, et al. Diagnostic yield and adverse effects of MRI‐guided free‐hand brain biopsies through a mini‐burr hole in dogs with encephalitis. J Vet Intern Med 2012;26:969–976. [DOI] [PubMed] [Google Scholar]
- 85. Harari J, Moore MM, Leathers CW, et al. Computed tomographic‐guided free‐hand needle biopsy of brain tumors in dogs. Prog Vet Neurol 1993;4:41–44. [Google Scholar]
- 86. Klopp LS, Ridgway M. Use of an endoscope in minimally invasive lesion biopsy and removal within the skull and cranial vault in two dogs and one cat. J Am Vet Med Assoc 2009;234:1573–1577. [DOI] [PubMed] [Google Scholar]
- 87. Coffey RJ, Lunsford LD. Animal research stereotactic instrument modified for computed tomographic guidance. Appl Neurophysiol 1987;50:81–86. [DOI] [PubMed] [Google Scholar]
- 88. Maciunas RJ, Galloway RL. Magnetic resonance and computed tomographic image‐directed stereotaxy for animal research. Stereotact Funct Neurosurg 1989;53:197–201. [DOI] [PubMed] [Google Scholar]
- 89. Koblik PD, LeCouteur RA, Higgins RJ, et al. CT‐guided brain biopsy using a modified Pelorus Mark III stereotactic system: Experience with 50 dogs. Vet Radiol Ultrasound 1999;40:434–440. [DOI] [PubMed] [Google Scholar]
- 90. Koblik PD, LeCouteur RA, Higgins RJ, et al. Modification and application of a Pelorus Mark III stereotactic system for CT‐guided brain biopsy in 50 dogs. Vet Radiol Ultrasound 1999;40:424–433. [DOI] [PubMed] [Google Scholar]
- 91. Moissonnier P, Blot S, Devauchelle P, et al. Stereotactic CT‐guided brain biopsy in the dog. J Small Anim Pract 2002;43:115–123. [DOI] [PubMed] [Google Scholar]
- 92. Troxel MT, Vite CH. CT‐guided stereotactic brain biopsy using the Kopf stereotactic system. Vet Radiol Ultrasound 2008;49:438–443. [DOI] [PubMed] [Google Scholar]
- 93. Packer RA, Freeman LJ, Miller MA, et al. Evaluation of minimally invasive excisional brain biopsy and intracranial brachytherapy catheter placement in dogs. Am J Vet Res 2011;72:109–121. [DOI] [PubMed] [Google Scholar]
- 94. Flegel T, Podell M, March PA, et al. Use of a disposable real‐time CT stereotactic navigator device for minimally invasive dog brain biopsy through a mini‐burr hole. AJNR Am J Neuroradiol 2002;23:1160–1163. [PMC free article] [PubMed] [Google Scholar]
- 95. Taylor AR, Cohen ND, Fletcher S, et al. Application and machine accuracy of a new frameless computed tomography‐guided stereotactic brain biopsy system in dogs. Vet Radiol Ultrasound 2013;54:332–342. [DOI] [PubMed] [Google Scholar]
- 96. Chen AV, Wininger FA, Frey S, et al. Description and validation of a magnetic resonance imaging‐guided stereotactic brain biopsy device in the dog. Vet Radiol Ultrasound 2012;53:150–156. [DOI] [PubMed] [Google Scholar]
- 97. Carvalho LH, Smirnov I, Baia GS, et al. Molecular signatures define two main classes of meningiomas. Mol Cancer 2007;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cancer Genome Atlas Research N . Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008;455:1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010;17:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high‐grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006;9:157–173. [DOI] [PubMed] [Google Scholar]
- 101. Shen R, Mo Q, Schultz N, et al. Integrative subtype discovery in glioblastoma using iCluster. PLoS One 2012;7:e35236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Brennan C, Momota H, Hambardzumyan D, et al. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One 2009;4:e7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Huse JT, Holland E, DeAngelis LM. Glioblastoma: Molecular analysis and clinical implications. Annu Rev Med 2013;64:59–70. [DOI] [PubMed] [Google Scholar]
- 104. Tanaka S, Louis DN, Curry WT, et al. Diagnostic and therapeutic avenues for glioblastoma: No longer a dead end? Nat Rev Clin Oncol 2013;10:14–26. [DOI] [PubMed] [Google Scholar]
- 105. Weller M, Stupp R, Hegi M, et al. Individualized targeted therapy for glioblastoma: Fact or fiction? Cancer J 2012;18:40–44. [DOI] [PubMed] [Google Scholar]
- 106. Betensky RA, Louis DN, Cairncross JG. Influence of unrecognized molecular heterogeneity on randomized clinical trials. J Clin Oncol 2002;20:2495–2499. [DOI] [PubMed] [Google Scholar]
- 107. Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science 2013;339:1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dickinson PJ, Roberts BN, Higgins RJ, et al. Expression of receptor tyrosine kinases VEGFR‐1 (FLT‐1), VEGFR‐2 (KDR), EGFR‐1, PDGFRalpha and c‐Met in canine primary brain tumours. Vet Comp Oncol 2006;4:132–140. [DOI] [PubMed] [Google Scholar]
- 109. Higgins RJ, Dickinson PJ, Lecouteur RA, et al. Spontaneous canine gliomas: Overexpression of EGFR, PDGFRalpha and IGFBP2 demonstrated by tissue microarray immunophenotyping. J Neurooncol 2009;98:49–55. [DOI] [PubMed] [Google Scholar]
- 110. Stoica G, Kim HT, Hall DG, et al. Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet Pathol 2004;41:10–19. [DOI] [PubMed] [Google Scholar]
- 111. Ide T, Uchida K, Kikuta F, et al. Immunohistochemical characterization of canine neuroepithelial tumors. Vet Pathol 2010;47:741–750. [DOI] [PubMed] [Google Scholar]
- 112. Lipsitz D, Higgins RJ, Kortz GD, et al. Glioblastoma multiforme: Clinical findings, magnetic resonance imaging, and pathology in five dogs. Vet Pathol 2003;40:659–669. [DOI] [PubMed] [Google Scholar]
- 113. Dickinson PJ, Sturges BK, Higgins RJ, et al. Vascular endothelial growth factor mRNA expression and peritumoral edema in canine primary central nervous system tumors. Vet Pathol 2008;45:131–139. [DOI] [PubMed] [Google Scholar]
- 114. Platt SR, Scase TJ, Adams V, et al. Vascular endothelial growth factor expression in canine intracranial meningiomas and association with patient survival. J Vet Intern Med 2006;20:663–668. [DOI] [PubMed] [Google Scholar]
- 115. Matiasek LA, Platt SR, Adams V, et al. Ki‐67 and vascular endothelial growth factor expression in intracranial meningiomas in dogs. J Vet Intern Med 2009;23:146–151. [DOI] [PubMed] [Google Scholar]
- 116. Rossmeisl JH, Duncan RB, Huckle WR, et al. Expression of vascular endothelial growth factor in tumors and plasma from dogs with primary intracranial neoplasms. Am J Vet Res 2007;68:1239–1245. [DOI] [PubMed] [Google Scholar]
- 117. Mandara MT, Ricci G, Rinaldi L, et al. Immunohistochemical identification and image analysis quantification of oestrogen and progesterone receptors in canine and feline meningioma. J Comp Pathol 2002;127:214–218. [DOI] [PubMed] [Google Scholar]
- 118. Theon AP, Lecouteur RA, Carr EA, et al. Influence of tumor cell proliferation and sex‐hormone receptors on effectiveness of radiation therapy for dogs with incompletely resected meningiomas. J Am Vet Med Assoc 2000;216:701–707. [DOI] [PubMed] [Google Scholar]
- 119. Long S, Argyle DJ, Nixon C, et al. Telomerase reverse transcriptase (TERT) expression and proliferation in canine brain tumours. Neuropathol Appl Neurobiol 2006;32:662–673. [DOI] [PubMed] [Google Scholar]
- 120. Mandrioli L, Panarese S, Cesari A, et al. Immunohistochemical expression of h‐telomerase reverse transcriptase in canine and feline meningiomas. J Vet Sci 2007;8:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mandara MT, Pavone S, Mandrioli L, et al. Matrix metalloproteinase‐2 and matrix metalloproteinase‐9 expression in canine and feline meningioma. Vet Pathol 2009;46:836–845. [DOI] [PubMed] [Google Scholar]
- 122. Beltran E, Matiasek K, Risio LD, et al. Expression of MMP‐2 and MMP‐9 in benign canine rostrotentorial meningiomas is not correlated to the extent of peritumoral edema. Vet Pathol 2013;50:1091–1098. [DOI] [PubMed] [Google Scholar]
- 123. Platt S, Kent M, Northrup N, et al. Immunohistochemical quantification of interleukin‐6 and interleukin‐8 expression in canine intracranial meningiomas. J Vet Intern Med 2012;26:807. (abstract) [Google Scholar]
- 124. Ramos‐Vara JA, Miller MA, Gilbreath E, et al. Immunohistochemical detection of CD34, E‐cadherin, claudin‐1, glucose transporter 1, laminin, and protein gene product 9.5 in 28 canine and 8 feline meningiomas. Vet Pathol 2010;47:725–737. [DOI] [PubMed] [Google Scholar]
- 125. Mariani CJ. Matrix metalloproteinase‐2 and ‐9 in canine central nervous system tumors In: Proceedings of the American College of Veterinary Internal Medicine Forum, New Orleans, LA, May 30‐June 2, 2012:421. [Google Scholar]
- 126. Rossmeisl JH Jr, Robertson JL, Zimmerman KL, et al. Cyclooxygenase‐2 (COX‐2) expression in canine intracranial meningiomas. Vet Comp Oncol 2009;7:173–180. [DOI] [PubMed] [Google Scholar]
- 127. Gibo DM, Dickinson P, Robertson J, et al. Highly potent toxin targeting IL‐13Ra2 in canine and human glioblastoma. Neuro Oncol 2012;14:vi31–vi32. [Google Scholar]
- 128. Gibo DM, Dickinson P, Robertson J, et al. Interleukin 13 receptor alpha‐2 is widely over‐expressed in human and canine primary brain tumors as detected by novel bispecies‐specific monoclonal antibodies. Neuro Oncol 2012;14:vi47. [Google Scholar]
- 129. Debinski W, Dickinson P, Rossmeisl JH, et al. New agents for targeting of IL‐13RA2 expressed in primary human and canine brain tumors. PLoS One 2013;8:e77719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sturges BK, Dickinson PJ, Aina OH, et al. Identification of novel targeting peptides for canine glioma. J Vet Intern Med 2008;22:771. [Google Scholar]
- 131. Thomson SA, Kennerly E, Olby N, et al. Microarray analysis of differentially expressed genes of primary tumors in the canine central nervous system. Vet Pathol 2005;42:550–558. [DOI] [PubMed] [Google Scholar]
- 132. Dickinson PJ, Nagarajan RP, Lin D, et al. Genome wide methylation sequencing to profile epigenetic modifications in canine glioma In: Proceedings of the American College of Veterinary Internal Medicine, Seattle, WA, June 12‐15, 2013:343. [Google Scholar]
- 133. Courtay‐Cahen C, Platt SR, De Risio L, et al. Preliminary analysis of genomic abnormalities in canine meningiomas. Vet Comp Oncol 2008;6:182–192. [DOI] [PubMed] [Google Scholar]
- 134. Thomas R, Duke SE, Wang HJ, et al. ‘Putting our heads together’: Insights into genomic conservation between human and canine intracranial tumors. J Neurooncol 2009;94:333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Dickinson PJ, Surace EI, Cambell M, et al. Expression of the tumor suppressor genes NF2, 4.1B, and TSLC1 in canine meningiomas. Vet Pathol 2009;46:884–892. [DOI] [PubMed] [Google Scholar]
- 136. Reitman ZJ, Olby NJ, Mariani CL, et al. IDH1 and IDH2 hotspot mutations are not found in canine glioma. Int J Cancer 2010;127:245–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. York D, Higgins RJ, LeCouteur RA, et al. TP53 mutations in canine brain tumors. Vet Pathol 2012;49:796–801. [DOI] [PubMed] [Google Scholar]
- 138. Schwartz M, Lamb CR, Brodbelt DC, et al. Canine intracranial neoplasia: Clinical risk factors for development of epileptic seizures. J Small Anim Pract 2011;52:632–637. [DOI] [PubMed] [Google Scholar]
- 139. Foster ES, Carrillo JM, Patnaik AK. Clinical signs of tumors affecting the rostral cerebrum in 43 dogs. J Vet Intern Med 1988;2:71–74. [DOI] [PubMed] [Google Scholar]
- 140. Heidner GL, Kornegay JN, Page RL, et al. Analysis of survival in a retrospective study of 86 dogs with brain tumors. J Vet Intern Med 1991;5:219–226. [DOI] [PubMed] [Google Scholar]
- 141. Turrel JM, Fike JR, LeCouteur RA, et al. Radiotherapy of brain tumors in dogs. J Am Vet Med Assoc 1984;184:82–86. [PubMed] [Google Scholar]
- 142. Van Meervenne S, Verhoeven PS, de Vos J, et al. Comparison between symptomatic treatment and lomustine supplementation in 71 dogs with intracranial, space‐occupying lesions. Vet Comp Oncol 2012;12:67–77. [DOI] [PubMed] [Google Scholar]
- 143. Rossmeisl JH Jr, Jones JC, Zimmerman KL, et al. Survival time following hospital discharge in dogs with palliatively treated primary brain tumors. J Am Vet Med Assoc 2013;242:193–198. [DOI] [PubMed] [Google Scholar]
- 144. Kostolich M, Dulisch ML. A surgical approach to the canine olfactory bulb for meningioma removal. Vet Surg 1987;16:273–277. [DOI] [PubMed] [Google Scholar]
- 145. Axlund TW, McGlasson ML, Smith AN. Surgery alone or in combination with radiation therapy for treatment of intracranial meningiomas in dogs: 31 cases (1989–2002). J Am Vet Med Assoc 2002;221:1597–1600. [DOI] [PubMed] [Google Scholar]
- 146. Rossmeisl J. Craniectomy for the treatment of canine meningiomas In: Proceedings of the American College of Veterinary Surgery Symposium, Washington, DC, October 9‐12, 2003. [Google Scholar]
- 147. Greco JJ, Aiken SA, Berg JM, et al. Evaluation of intracranial meningioma resection with a surgical aspirator in dogs: 17 cases (1996–2004). J Am Vet Med Assoc 2006;229:394–400. [DOI] [PubMed] [Google Scholar]
- 148. Klopp LS, Rao S. Endoscopic‐assisted intracranial tumor removal in dogs and cats: Long‐term outcome of 39 cases. J Vet Intern Med 2009;23:108–115. [DOI] [PubMed] [Google Scholar]
- 149. Gordon LE, Thacher C, Matthiesen DT, et al. Results of craniotomy for the treatment of cerebral meningioma in 42 cats. Vet Surg 1994;23:94–100. [DOI] [PubMed] [Google Scholar]
- 150. Gallagher JG, Berg J, Knowles KE, et al. Prognosis after surgical excision of cerebral meningiomas in cats: 17 cases (1986–1992). J Am Vet Med Assoc 1993;203:1437–1440. [PubMed] [Google Scholar]
- 151. Troxel MT, Vite CH, Van Winkle TJ, et al. Feline intracranial neoplasia: Retrospective review of 160 cases (1985–2001). J Vet Intern Med 2003;17:850–859. [DOI] [PubMed] [Google Scholar]
- 152. Brearley MJ, Jeffery ND, Phillips SM, et al. Hypofractionated radiation therapy of brain masses in dogs: A retrospective analysis of survival of 83 cases (1991–1996). J Vet Intern Med 1999;13:408–412. [DOI] [PubMed] [Google Scholar]
- 153. Niebauer GW, Dayrell‐Hart BL, Speciale J. Evaluation of craniotomy in dogs and cats. J Am Vet Med Assoc 1991;198:89–95. [PubMed] [Google Scholar]
- 154. Fulton LM, Steinberg HS. Preliminary study of lomustine in the treatment of intracranial masses in dogs following localization by imaging techniques. Semin Vet Med Surg (Small Anim) 1990;5:241–245. [PubMed] [Google Scholar]
- 155. Meij B, Voorhout G, Rijnberk A. Progress in transsphenoidal hypophysectomy for treatment of pituitary‐dependent hyperadrenocorticism in dogs and cats. Mol Cell Endocrinol 2002;197:89–96. [DOI] [PubMed] [Google Scholar]
- 156. Kent MS, Bommarito D, Feldman E, et al. Survival, neurologic response, and prognostic factors in dogs with pituitary masses treated with radiation therapy and untreated dogs. J Vet Intern Med 2007;21:1027–1033. [DOI] [PubMed] [Google Scholar]
- 157. Theon AP, Feldman EC. Megavoltage irradiation of pituitary macrotumors in dogs with neurologic signs. J Am Vet Med Assoc 1998;213:225–231. [PubMed] [Google Scholar]
- 158. Hirano T, Nakagawa A, Uenohara H, et al. Pulsed liquid jet dissector using holmium:YAG laser—A novel neurosurgical device for brain incision without impairing vessels. Acta Neurochir (Wien) 2003;145:401–406; discussion 406. [DOI] [PubMed] [Google Scholar]
- 159. Feder BM, Fry TR, Kostolich M, et al. Nd:YAG laser cytoreduction of an invasive intracranial meningioma in a dog. Prog Vet Neurol 1993;4:3–9. [Google Scholar]
- 160. Schwartz JA, Shetty AM, Price RE, et al. Feasibility study of particle‐assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res 2009;69:1659–1667. [DOI] [PubMed] [Google Scholar]
- 161. Ellis TL, Garcia PA, Rossmeisl JH Jr, et al. Nonthermal irreversible electroporation for intracranial surgical applications. Laboratory investigation. J Neurosurg 2011;114:681–688. [DOI] [PubMed] [Google Scholar]
- 162. Garcia PA, Pancotto T, Rossmeisl JH Jr, et al. Non‐thermal irreversible electroporation (N‐TIRE) and adjuvant fractionated radiotherapeutic multimodal therapy for intracranial malignant glioma in a canine patient. Technol Cancer Res Treat 2011;10:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Herickhoff CD, Light ED, Bing KF, et al. Dual‐mode intracranial catheter integrating 3D ultrasound imaging and hyperthermia for neuro‐oncology: Feasibility study. Ultrason Imaging 2009;31:81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Chan F, Kassim I, Lo C, et al. Image‐guided robotic neurosurgery—An in vitro and in vivo point accuracy evaluation experimental study. Surg Neurol 2009;71:640–647; discussion 647–648. [DOI] [PubMed] [Google Scholar]
- 165. Li Y, Rey‐Dios R, Roberts DW, et al. Intraoperative fluorescence‐guided resection of high‐grade gliomas: A comparison of the present techniques and evolution of future strategies. World Neurosurg 2013. Jul 9. pii: S1878‐8750(13):00760–2. doi: 10.1016/j.wneu.2013.06.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 166. Jeffery N, Brearley MJ. Brain tumors in the dog: Treatment of 10 cases and review of recent literature. J Small Anim Pract 1993;34:367–372. [Google Scholar]
- 167. Dimski DS, Cook JR. Carmustine‐induced partial remission of an astrocytoma in a dog. J Am Anim Hosp Assoc 1990;26:179–182. [Google Scholar]
- 168. Jung DI, Kim HJ, Park C, et al. Long‐term chemotherapy with lomustine of intracranial meningioma occurring in a miniature schnauzer. J Vet Med Sci 2006;68:383–386. [DOI] [PubMed] [Google Scholar]
- 169. Tamura S, Tamura Y, Ohoka A, et al. A canine case of skull base meningioma treated with hydroxyurea. J Vet Med Sci 2007;69:1313–1315. [DOI] [PubMed] [Google Scholar]
- 170. Boudreau CE, York D, Higgins RJ, et al. Investigation of molecular signaling pathways in canine primary gliomas. J Vet Intern Med 2013;27:675. [Google Scholar]
- 171. Kim L, Glantz M. Chemotherapeutic options for primary brain tumors. Curr Treat Options Oncol 2006;7:467–478. [DOI] [PubMed] [Google Scholar]
- 172. Matiasek K. Drug resistance—Does it matter in human and canine brain tumors? In: Proceedings of the American College of Veterinary Internal Medicine Forum, Denver, CO, June 15‐18, 2011:389–390. [Google Scholar]
- 173. Wick W, Meisner C, Hentschel B, et al. Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology 2013;81(17):1515–1522. [DOI] [PubMed] [Google Scholar]
- 174. Matiasek LA, Schlegel J, Starkey MP, et al. Testing epigenetic concepts in canine neurooncology I: Methylation of DNA‐repair enzyme o6‐methylguanin‐DNA‐methyltransferase In: Proceedings of the European Society of Veterinary Neurology/European College of Veterinary Neurology Neurooncology Symposium, Bologna, Italy, September 24‐26, 2009:39–40. [Google Scholar]
- 175. Juratli TA, Schackert G, Krex D. Current status of local therapy in malignant gliomas—A clinical review of three selected approaches. Pharmacol Ther 2013;139:341–358. [DOI] [PubMed] [Google Scholar]
- 176. Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine spontaneous glioma: A translational model system for convection‐enhanced delivery. Neuro Oncol 2010;12:928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bley CR, Sumova A, Roos M, et al. Irradiation of brain tumors in dogs with neurologic disease. J Vet Intern Med 2005;19:849–854. [DOI] [PubMed] [Google Scholar]
- 178. Evans SM, Dayrell‐Hart B, Powlis W, et al. Radiation therapy of canine brain masses. J Vet Intern Med 1993;7:216–219. [DOI] [PubMed] [Google Scholar]
- 179. Spugnini EP, Thrall DE, Price GS, et al. Primary irradiation of canine intracranial masses. Vet Radiol Ultrasound 2000;41:377–380. [DOI] [PubMed] [Google Scholar]
- 180. Norman A, Ingram M, Skillen RG, et al. X‐ray phototherapy for canine brain masses. Radiat Oncol Investig 1997;5:8–14. [DOI] [PubMed] [Google Scholar]
- 181. Ostertag CB, Warnke P, Kleihues P, et al. Iodine‐125 interstitial irradiation of virally induced dog brain tumours. Neurol Res 1984;6:176–180. [DOI] [PubMed] [Google Scholar]
- 182. Stubbs JB, Frankel RH, Schultz K, et al. Preclinical evaluation of a novel device for delivering brachytherapy to the margins of resected brain tumor cavities. J Neurosurg 2002;96:335–343. [DOI] [PubMed] [Google Scholar]
- 183. Liu BL, Cheng JX, Zhang X, et al. Controversies concerning the application of brachytherapy in central nervous system tumors. J Cancer Res Clin Oncol 2010;136:173–185. [DOI] [PubMed] [Google Scholar]
- 184. Schwarz SB, Thon N, Nikolajek K, et al. Iodine‐125 brachytherapy for brain tumours—a review. Radiat Oncol 2012;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Minniti G, Amichetti M, Enrici RM. Radiotherapy and radiosurgery for benign skull base meningiomas. Radiat Oncol 2009;4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Villavicencio AT, Burneikiene S, Romanelli P, et al. Survival following stereotactic radiosurgery for newly diagnosed and recurrent glioblastoma multiforme: A multicenter experience. Neurosurg Rev 2009;32:417–424. [DOI] [PubMed] [Google Scholar]
- 187. Lester NV, Hopkins AL, Bova FJ, et al. Radiosurgery using a stereotactic headframe system for irradiation of brain tumors in dogs. J Am Vet Med Assoc 2001;219:1562–1567, 1550. [DOI] [PubMed] [Google Scholar]
- 188. Mariani CL, Schubert TA, House RA, et al. Frameless stereotactic radiosurgery for the treatment of primary intracranial tumours in dogs. Vet Comp Oncol 2013. Sep 6. doi: 10.1111/vco.12056. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 189. Charney S, Witten M, Ettinger S, et al. CyberKnife radiosurgery for irradiation of tumors in dogs and cats. Vet Comp Oncol 2011;9:e42. [Google Scholar]
- 190. Kent MS. Stereotactic radiosurgery for brain tumors: Clinical trial update In: Proceedings of the American College of Veterinary Medicine Specialty Symposium (Pre‐Forum Neurology), New Orleans, LA, May 30‐June 2, 2012:25–26. [Google Scholar]
- 191. Larue SM. Advances in radiotherapy of neurological tumors In: Proceedings of the American College of Veterinary Internal Medicine Forum, Seattle, WA, June 12‐15, 2013:426–427. [Google Scholar]
- 192. Kraft SL, Gavin PR, Leathers CW, et al. Biodistribution of boron in dogs with spontaneous intracranial tumors following borocaptate sodium administration. Cancer Res 1994;54:1259–1263. [PubMed] [Google Scholar]
- 193. Bagley RS, Gavin PR, Moore MP, et al. Survival after BNCT in combination with surgery for dogs with spontaneous brain tumors In: Hawthorn MF, Shelly K, Wiersema RJ, eds. Frontiers in Neutron Capture Therapy, Vols 1 and 2. New York, NY: Kluwer Academic/Plenum Publ; 2001:1257–1261. [Google Scholar]
- 194. Nakamura H. Boron lipid‐based liposomal boron delivery system for neutron capture therapy: Recent development and future perspective. Future Med Chem 2013;5:715–730. [DOI] [PubMed] [Google Scholar]
- 195. Bobo RH, Laske DW, Akbasak A, et al. Convection‐enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 1994;91:2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Sampson JH, Brady ML, Petry NA, et al. Intracerebral infusate distribution by convection‐enhanced delivery in humans with malignant gliomas: Descriptive effects of target anatomy and catheter positioning. Neurosurgery 2007;60:ONS89‐98; discussion ONS98‐89. [DOI] [PubMed] [Google Scholar]
- 197. Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine model of convection‐enhanced delivery of liposomes containing CPT‐11 monitored with real‐time magnetic resonance imaging: Laboratory investigation. J Neurosurg 2008;108:989–998. [DOI] [PubMed] [Google Scholar]
- 198. Platt S, Nduom E, Kent M, et al. Canine model of convection‐enhanced delivery of cetuximab‐conjugated iron‐oxide nanoparticles monitored with magnetic resonance imaging. Clin Neurosurg 2012;59:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Kaluzova M, Platt SR, Kent M, et al. Targeted therapy of glioma stem cells using cetuximab‐conjugated IONPs. J Clin Invest 2014. In press. [Google Scholar]
- 200. Hood RL, Andriani RT Jr, Emch S, et al. Fiberoptic microneedle device facilitates volumetric infusate dispersion during convection‐enhanced delivery in the brain. Lasers Surg Med 2013;45:418–426. [DOI] [PubMed] [Google Scholar]
- 201. Robbins J, Dickinson PJ, York D, et al. Evaluation of delivery of retroviral replicating vector TOCA 511 in spontaneous canine brain tumor. Neuro Oncol 2012;14:vi48. [Google Scholar]
- 202. Dickinson PJ. TOCA‐511 gene therapy clinical trial update In: Proceedings of the American College of Veterinary Internal Medicine Specialty Symposium (Pre‐Forum Neurology), New Orleans, LA, May 30‐June 2, 2012:29–31. [Google Scholar]
- 203. Tobias A, Ahmed A, Moon KS, et al. The art of gene therapy for glioma: A review of the challenging road to the bedside. J Neurol Neurosurg Psychiatry 2013;84:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Garcia‐Escudero V, Gargini R, Izquierdo M. Glioma regression in vitro and in vivo by a suicide combined treatment. Mol Cancer Res 2008;6:407–417. [DOI] [PubMed] [Google Scholar]
- 205. Chauvet AE, Kesava PP, Goh CS, et al. Selective intraarterial gene delivery into a canine meningioma. J Neurosurg 1998;88:870–873. [DOI] [PubMed] [Google Scholar]
- 206. Pluhar GE, Grogan PT, Seiler C, et al. Anti‐tumor immune response correlates with neurological symptoms in a dog with spontaneous astrocytoma treated by gene and vaccine therapy. Vaccine 2010;28:3371–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Candolfi M, Kroeger KM, Pluhar GE, et al. Adenoviral‐mediated gene transfer into the canine brain in vivo. Neurosurgery 2007;60:167–177; discussion 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Candolfi M, Pluhar GE, Kroeger K, et al. Optimization of adenoviral vector‐mediated transgene expression in the canine brain in vivo, and in canine glioma cells in vitro. Neuro Oncol 2007;9:245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Oh S, Pluhar GE, McNeil EA, et al. Efficacy of nonviral gene transfer in the canine brain. J Neurosurg 2007;107:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Traas AM, Wang P, Ma X, et al. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol Ther 2007;15:1423–1431. [DOI] [PubMed] [Google Scholar]
- 211. Springer SL, Vite CH, Polesky AC, et al. Infection and establishment of latency in the dog brain after direct inoculation of a nonpathogenic strain of herpes simplex virus‐1. J Neurovirol 2001;7:149–154. [DOI] [PubMed] [Google Scholar]
- 212. Haurigot V, Marco S, Ribera A, et al. Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J Clin Invest 2013;123:3254–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Ciron C, Desmaris N, Colle MA, et al. Gene therapy of the brain in the dog model of Hurler's syndrome. Ann Neurol 2006;60:204–213. [DOI] [PubMed] [Google Scholar]
- 214. Jimenez D, Higgins R, LeCouteur R, et al. Bystander killing in canine meningioma cells with a recombinant adeno‐associated virus vector containing herpes simplex viral thymidine kinase [rAAV HSV‐tk]. Vet Pathol 1998;35:443. [Google Scholar]
- 215. Ignatova TN, Kukekov VG, Laywell ED, et al. Human cortical glial tumors contain neural stem‐like cells expressing astroglial and neuronal markers in vitro. Glia 2002;39:193–206. [DOI] [PubMed] [Google Scholar]
- 216. Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 2006;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature 2004;432:396–401. [DOI] [PubMed] [Google Scholar]
- 218. Wu X, Rauch TA, Zhong X, et al. CpG island hypermethylation in human astrocytomas. Cancer Res 2010;70:2718–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Blacking TM, Waterfall M, Argyle DJ. CD44 is associated with proliferation, rather than a specific cancer stem cell population, in cultured canine cancer cells. Vet Immunol Immunopathol 2011;141:46–57. [DOI] [PubMed] [Google Scholar]
- 220. Stoica G, Lungu G, Martini‐Stoica H, et al. Identification of cancer stem cells in dog glioblastoma. Vet Pathol 2009;46:391–406. [DOI] [PubMed] [Google Scholar]
- 221. York D, Higgins RJ, LeCouteur RA, et al. Characterization of a novel canine GBM‐derived cell line (G06A) and orthotopic tumors in mice. J Vet Intern Med 2012;26:808. [Google Scholar]
- 222. York D, Higgins RJ, LeCouteur RA, et al. A novel anti‐canine CD133 antibody colocalizes with GFAP in normal and neoplastic brain tissue. J Vet Intern Med 2012;26:807. [Google Scholar]
- 223. Lynch S, Pang L, Argyle D. Identification of cancer stem cells in canine glioma and resistance to therapeutic modalities. Vet Comp Oncol 2011;9:e1. [Google Scholar]
- 224. Pennington ME, Lam KS, Cress AE. The use of a combinatorial library method to isolate human tumor cell adhesion peptides. Mol Divers 1996;2:19–28. [DOI] [PubMed] [Google Scholar]
- 225. Zwingenberger AL, Kent MS, Shi C, et al. Affinity of the alpha4‐beta1 integrin‐targeting peptide LLP2A to canine lymphoma. Vet Immunol Immunopathol 2012;145:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Aina OH, Maeda Y, Harrison M, et al. Canine malignant melanoma alpha‐3 integrin binding peptides. Vet Immunol Immunopathol 2011;143:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Xiao W, Yao N, Peng L, et al. Near‐infrared optical imaging in glioblastoma xenograft with ligand‐targeting alpha 3 integrin. Eur J Nucl Med Mol Imaging 2009;36:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Zwingenberger AL, Kent MS, Liu R, et al. In‐vivo biodistribution and safety of 99mTc‐LLP2A‐HYNIC in canine non‐Hodgkin lymphoma. PLoS One 2012;7:e34404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Zwingenberger AL, Kent MS, Liu R, et al. Targeted imaging of the alpha4‐beta1 integrin in canine lymphoma. Vet Radiol Ultrasound 2010;51:577. [Google Scholar]
- 230. Assi H, Candolfi M, Baker G, et al. Gene therapy for brain tumors: Basic developments and clinical implementation. Neurosci Lett 2012;527:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Jackson C, Ruzevick J, Brem H, et al. Vaccine strategies for glioblastoma: Progress and future directions. Immunotherapy 2013;5:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Sioka C, Kyritsis AP. Chemotherapy, hormonal therapy, and immunotherapy for recurrent meningiomas. J Neurooncol 2009;92:1–6. [DOI] [PubMed] [Google Scholar]
- 233. Marsh JC, Goldfarb J, Shafman TD, et al. Current status of immunotherapy and gene therapy for high‐grade gliomas. Cancer Control 2013;20:43–48. [DOI] [PubMed] [Google Scholar]
- 234. Boozer LB, Davis TW, Borst LB, et al. Characterization of immune cell infiltration into canine intracranial meningiomas. Vet Pathol 2012;49:784–795. [DOI] [PubMed] [Google Scholar]
- 235. Xiong W, Candolfi M, Liu C, et al. Human Flt3L generates dendritic cells from canine peripheral blood precursors: Implications for a dog glioma clinical trial. PLoS ONE 2010;5:e11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Pluhar GE. Canine brain tumor clinical trials at the University of Minnesota In: Proceedings of the American College of Veterinary Internal Medicine Specialty Symposium (Pre‐Forum Neurology), Denver, CO, June 15‐18, 2011:29–31. [Google Scholar]
- 237. Andersen BM, Pluhar GE, Seiler C, et al. Vaccination for invasive canine meningioma induces in situ production of antibodies capable of antibody‐dependent cell‐mediated cytotoxicity. Cancer Res 2013;73:2987–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]


