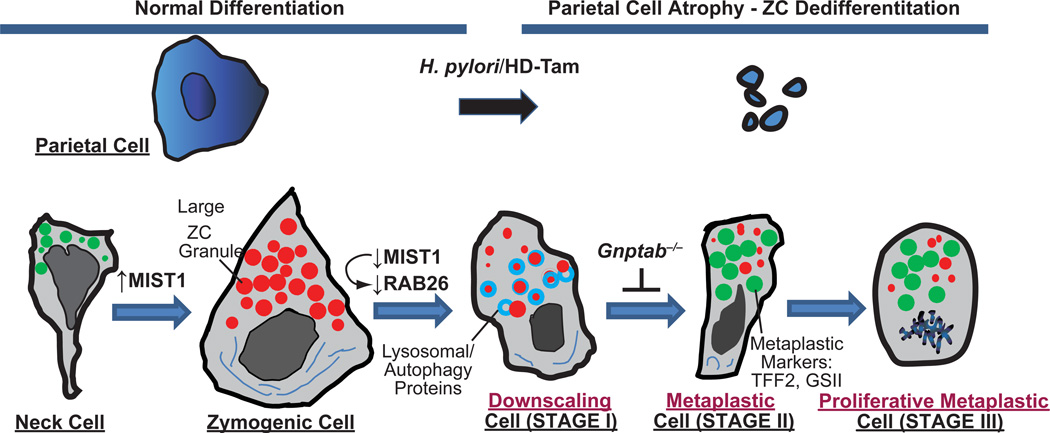
Fig. 2. Proposed stages of reprogramming and metaplasia in the stomach.
Cartoon based on our work and that of the literature proposing that reprogramming during metaplastic response to parietal cell damage in the stomach proceeds via 4 distinct stages. Zymogenic “Chief” Cells normally differentiate from a secretory progenitor, known as the mucous neck cell. Atrophy (death) of acid-secreting parietal cells (caused, e.g., by chronic infection with Helicobacter pylori or, in mouse models, by high doses of tamoxifen “HD-Tam”) causes chief cells to reprogram. We propose that the first stage of the reprogramming response is for Chief Cells to “downscale” their secretory architecture (including decreased digestive enzyme-containing granules, red, and dismantling of the elaborate rER network, dark blue) in a process involving decreased expression of the bHLH transcription factor, MIST1, which is required for maintaining apical secretory granules. Once large enzyme-containing granules are recycled, cells re-express progenitor genes (green) with much scanter digestive enzyme granules and are thus, by definition, metaplastic (called “SPEM” based on greatly increased expression of TFF2, aka Spasmolytic Polypeptide). In the third stage, cells reenter the cell cycle, at which point they may, via poorly understood processes, give rise to other metaplasias (like intestinal metaplasia in humans) or progress directly to cancer. In a parallel to pancreatic ADM, metaplastic chief cells may also regenerate other cells again via processes that are yet to be determined. Genes expressed during mucous neck, zymogenic, and metaplastic stages are delineated below, along with secreted factors at each of those stages.