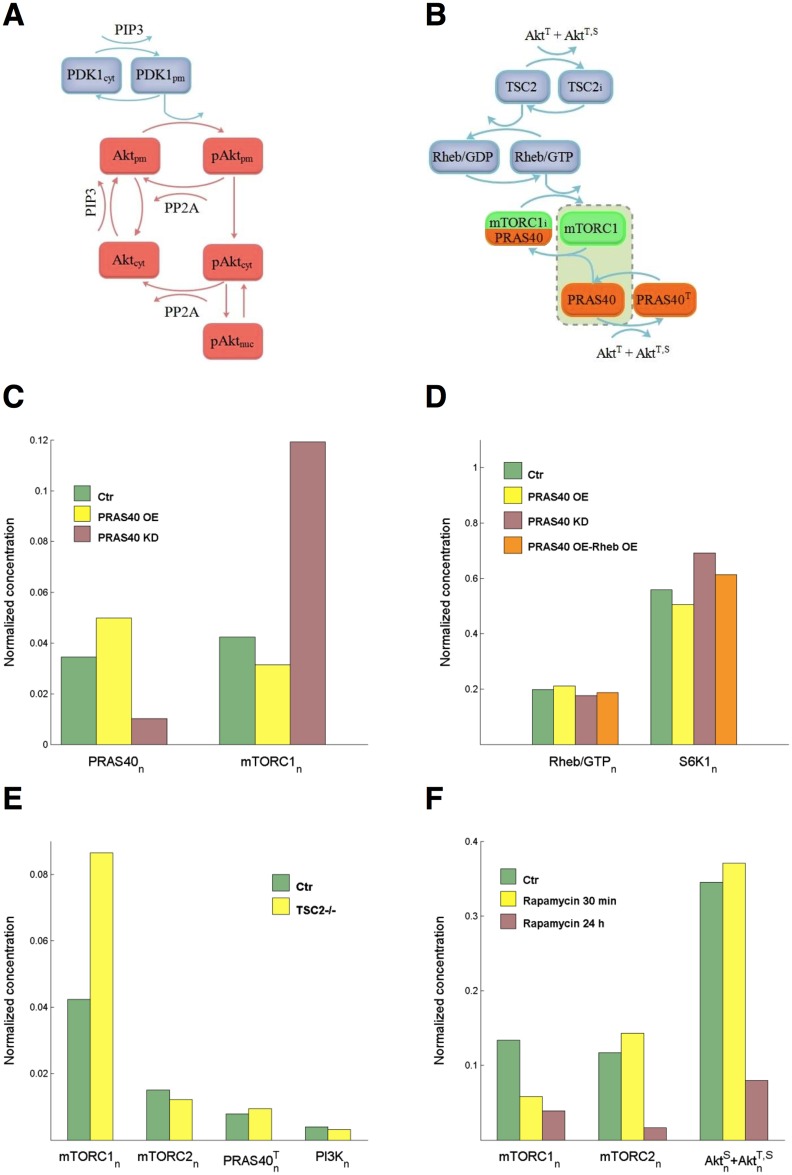
Fig 4. Improved modeling of Akt and mTOR.
(A) PIP3 recruits PDK1 and Akt to the plasma membrane. At the PM, Akt is phosphorylated by PDK1 and dephosphorylated by PP2A. Transport of not yet phosphorylated Akt from PM back to cytosol is regulated by the rate constant k−13. Phosphorylated Akt is transported to cytosol (rate constant kmc) where it is dephosphorylated by PP2A or imported into the nucleus (kcn). Export from nucleus is regulated by knc. (B) Phosphorylated Akt inactivates TSC2. Active TSC2 promotes Rheb binding to GDP and TSC2 inactivation stimulates the conversion from Rheb/GDP to active Rheb/GTP, which in turn activates mTORC1. mTORC1 is also inhibited by PRAS40. The box including active mTORC1 and proline-rich Akt substrate of 40 kDa (PRAS40) accounts for reaction (3) in S1 File (Text S3). (C) PRAS knockdown (KD: KmTOR increased tenfold compared to control and φ = 0.7, pink boxes) and overexpression (OE: KmTOR halved compared to control and φ = −5/6, yellow boxes) and effect on mTORC1 activation at 1 nM insulin. (D) Normalized concentrations Rheb/GTP and of T389 S6K1 at 1 nM insulin with PRAS knockdown (tenfold bPRAS decrease, pink boxes), PRAS overexpression (twofold bPRAS increase, yellow boxes), and with both PRAS (twofold bPRAS increase) and Rheb (fivefold bRheb increase) overexpression (orange boxes). (E) Normalized protein concentrations in TSC2-null cells at 1 nM insulin. (F) Response to short-term and long-term rapamycin treatment of mTORC1 and mTORC2, and effect on Akt phosphorylation at 10 nM insulin. Short- and long-term treatments: KmTOR in Eq (14) of S1 File (Text S3) set to 0.1 of control. Long-term-treatment: parameters and of Akt Eqs (9)–(11) set to 0.1 of control.