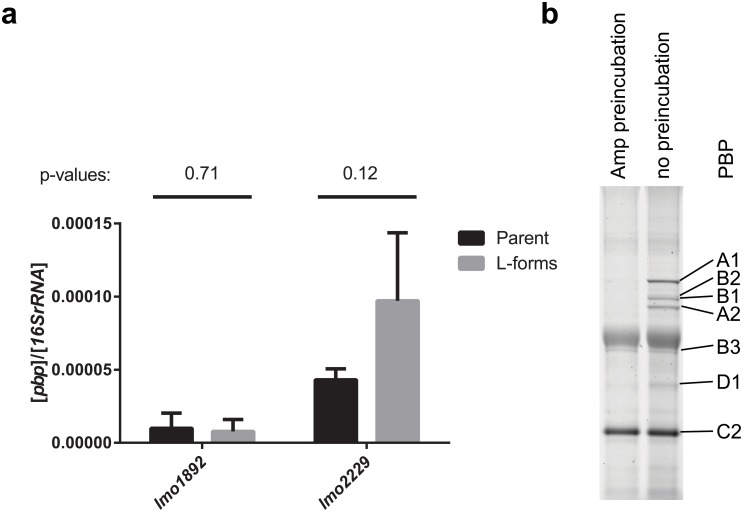
Fig 3. L-forms express both bifunctional PBPs.
(a) Transcription levels of lmo1892 (encoding PBP A1) and lmo2229 (encoding PBP A2) are not significantly different in parental and L-form cells as determined by quantitative real-time PCR. Means and standard deviations of three biological replicates (n = 3), as well as pairwise t-tests and the corresponding P-values are depicted. (b) Incubation of total cell extracts of L-form L. monocytogenes with fluorescent BOCILLIN FL reveals the presence of the two bifunctional PBPs, PBP A1 and A2, and the transpeptidases PBP B1, B2 and B3. In addition, PBP C2, containing a β-lactamase class C domain, and PBP D1, containing a DD-carboxypeptidase domain, were detected. Samples preincubated with high concentrations of ampicillin serve as specificity control of BOCILLIN FL binding. PBP C2 and B3 were already reported to be poorly competed by ampicillin [33].