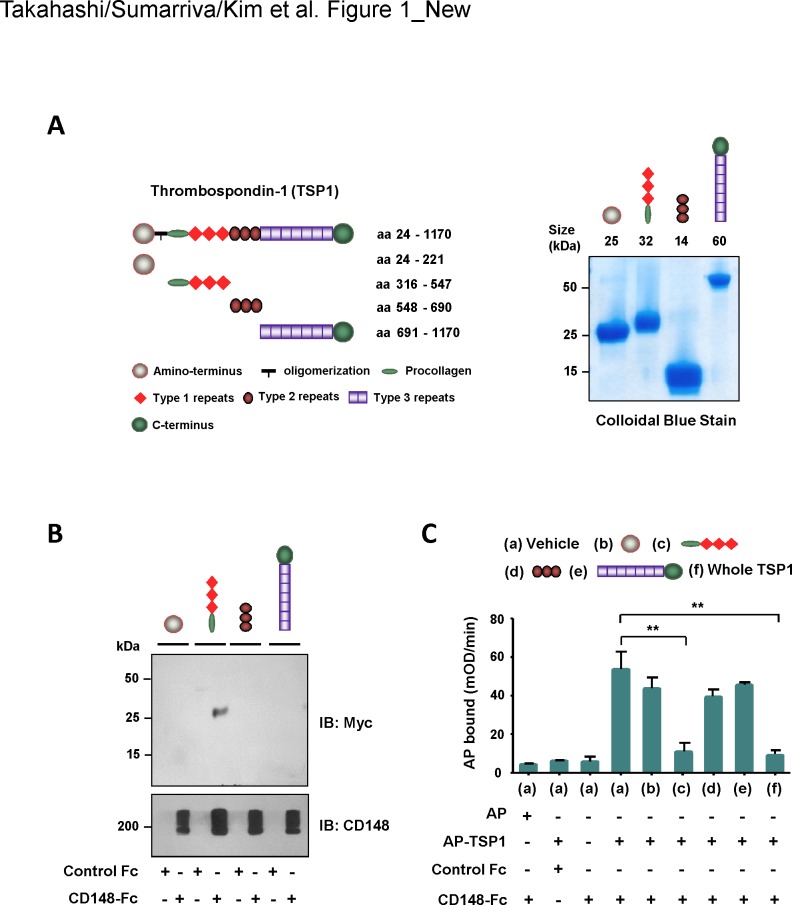
Fig 1. Assessment of CD148-interacting region in TSP1.
(A) Recombinant TSP1 fragments that correspond to the structural elements were prepared using HEK293E cells. Left panel shows a schematic representation of the TSP1 fragments. The number of amino acid residues includes the signal peptide sequence. Right panel shows colloidal blue stain of the purified TSP1 fragments. Twelve micrograms of protein were separated on a 10% polyacrylamide gel and stained with colloidal blue to assess size and purity. The expected size of protein is also shown. (B) TSP1 fragments (17 nM) were incubated with either 44 pmol of CD148-Fc or control Fc (Fc alone). Fc-proteins were pulled down with Protein-G beads and the binding of TSP1 fragments was assessed by immunoblotting using anti-Myc antibody (upper panel). The membrane was reprobed with anti-CD148 antibody to confirm the pull down of CD148-Fc (lower panel). Representative data of five independent experiments is shown. Note: The TSP1 fragment containing the procollagen domain and type 1 repeats binds to CD148-Fc. (C) Protein-A plates conjugated with CD148-Fc (11.3 nM) or equal molar of control Fc were incubated with AP-TSP1 or AP (12 nM) in the presence or absence of TSP1 fragments (25 nM) or whole TSP1 protein (25 nM). The bound AP-TSP1 was assessed by an AP activity assay. The data show mean ± SEM of quadruplicate determinations. Representative data of five independent experiments is shown. ** P < 0.05 Note: The binding of AP-TSP1 to CD148-Fc is blocked with either a TSP1 fragment containing the procollagen domain and type 1 repeats or whole TSP1 protein.