Figure 4. Pluripotency factors and X chromosome inactivation.
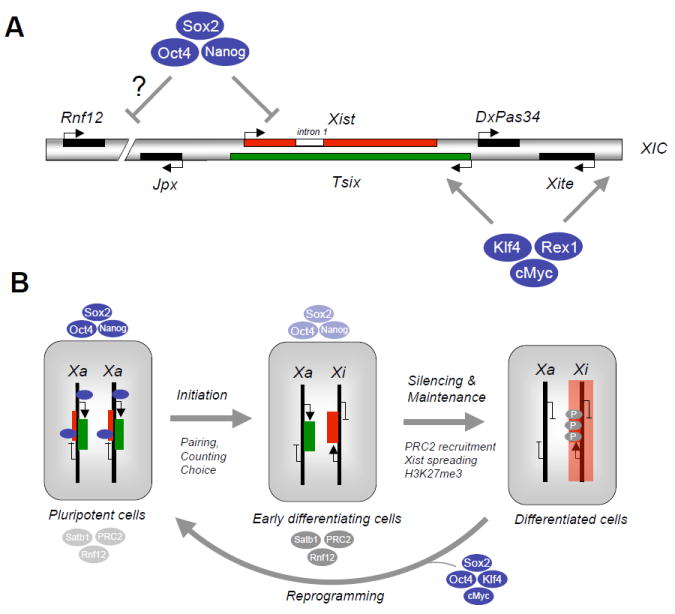
(A) Schematic depiction of X Inactivation Center (XIC) on the X chromosome with positions of non-coding RNAs Xist, Tsix, DxPas34, Xite, Jpx and the dose-dependent Xist activator Rnf12 as indicated. In undifferentiated female embryonic stem cells (ESCs), Xist (intron 1) and possibly Rnf12 are occupied and transcriptionally suppressed by Oct4, Sox2 and Nanog while Xite and Tsix are bound and transcriptionally activated by Klf4, Rex1 and cMyc. (B) In female ESCs, Xist is silenced while Tsix is activated by the pluripotency factors shown in (A). Upon differentiation, X chromosome inactivation ensues through a multistep process that involves initiation (pairing and counting of X chromosomes to choose the future inactive and active X, Xi and Xa), silencing and maintenance of the silenced X. The initiation and onset of silencing are tightly linked with the downregulation of pluripotency factors and the concomitant upregulation of chromatin regulators that mediate XCI, such as PRC2 (recruited by Xist RNA to mediate spreading of silencing along the entire X), and Satb1 (organization of active chromatin into loops). Silencing of the inactive X further results in H3K27 trimethylation by PRC2. Introduction of Oct4, Sox2, Klf4 and cMyc into differentiated cells gives rise to induced pluripotent stem cells, which is accompanied by X chromosome reactivation in mouse.
