Abstract
Background and objectives
We evaluated rates and factors associating with late referral (LR) and describe association of LR with access to renal transplantation and patient survival in children in the United Kingdom. Early requirement of RRT within 90 days of presentation to a pediatric nephrologist was classed as a LR, and those >90 days as an early referral (ER).
Design, setting, participants, & measurements
We included patients who commenced RRT, aged ≥3 months and <16 years, from 1996 to 2012.
Results
Of 1603 patients, 25.5% (n=408) were LR, of which 75% commenced RRT in <30 days following presentation. Those with LR were more likely to be older at presentation, female, and black. The primary renal disease in LR was more likely to be glomerular disease (odds ratio [OR], 1.6; 95% confidence interval [95% CI], 1.12 to 2.29), renal malignancy and associated diseases (OR, 4.11; 95% CI, 1.57 to 10.72), tubulo-interstitial diseases (OR, 2.37; 95% CI, 1.49 to 3.78), or an uncertain renal etiology (OR, 5.75; 95% CI, 3.1 to 10.65). Significant differences in rates of transplantation between LR and ER remained up to 1-year following commencement of dialysis (21% versus 61%, P<0.001) but with no differences for donor source (33.3% and 35.3% living donor in LR and ER respectively, P=0.55). The median (interquartile range) follow-up time was 4.8 years (2.9–7.6). There were 55 deaths with no statistically significant difference in survival in the LR group compared with the ER group (hazard ratio, 1.30; 95% CI, 0.7 to 2.3; P=0.40).
Conclusions
We found that 25% of children starting RRT in the United Kingdom receive a LR to pediatric renal services, with little change observed over the past two decades. Those with LR are unable to benefit from pre-emptive transplantation and require longer periods of dialysis before transplantation. There is an urgent need to understand causes of avoidable LR and develop strategies to improve kidney awareness more widely among health care professionals looking after children.
Keywords: chronic kidney disease, end stage kidney disease, child, late referral, follow-up studies, humans, kidney transplantation, living donors, renal dialysis, renal replacement therapy
Introduction
Adults with ESRD requiring RRT are often categorized by the time interval between starting RRT and the time they are first seen by a nephrologist (1,2). Those starting RRT within 90 days following first presentation to a nephrologist are typically described as a late referral (LR) (1–6), with other definitions of LR ranging from 1 to 6 months (6–9).
LR in adults is common with estimates of between 20% and 50% (10–15), and has been shown to adversely affect dialysis access (3,7,9), hemoglobin levels (3,4,9), and choice of modality at the start of RRT (3,5,8), as well as being associated with poorer survival (4,5,7,9) and prolonged hospital stay (8). LR will also delay listing for transplantation as work-up is delayed and reduce the chance of pre-emptive transplantation (16,17). Published data regarding the prevalence or impact of LR in children is scarce (18–21); as are any United Kingdom-specific recommendations regarding optimal timing of referral for children with CKD to a pediatric nephrologist.
Available guidelines for referral of children from North America with CKD (based on level of renal dysfunction) suggest that children with signs of CKD or GFR<60 ml/min per 1.73 m2 should be referred to a pediatric nephrologist (22), and referral is essential for those with GFR<30 ml/min per 1.73 m2 (22).
Early requirement of RRT within 90 days of presentation in children was termed as LR in this study. In the absence of any existing published United Kingdom data, this registry-based study of the pediatric UK Renal Registry (UKRR) database aimed: (1) to describe rates and factors influencing LRs in the United Kingdom pediatric RRT population and (2) to investigate the association of LR with access to renal transplantation and patient survival in the United Kingdom over the past 17 years.
Materials and Methods
Study Population
All children on the UKRR pediatric database who commenced RRT aged ≥3 months and <16 years in the United Kingdom between January 1, 1996 and December 31, 2012 (n=1741) were eligible. Patients were identified following a retrospective review of the pediatric database and include data returns from all 13 United Kingdom tertiary pediatric nephrology centers providing RRT. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the 'Declaration of Istanbul on Organ Trafficking and Transplant Tourism'.
Data collected included: demographic characteristics including date and age at first review with pediatric nephrologist, gender, ethnicity, date and modality at start of RRT, eGFR at presentation and at start of RRT, primary renal diagnosis (PRD), date of transplantation, and date of death. GFR was estimated from serum creatinine using the new Schwartz formula (23).
Children were classified as receiving a LR if the time interval between first review with a pediatric nephrologist and commencement of RRT was <90 days; those where the interval was ≥90 days were categorized as early referral (ER). Children aged <3 months at the start of RRT were excluded from the analyses as they would all be classified as LR by definition. Date at start of RRT was split into three groups (1996–2001, 2002–2007, and 2008–2012) as ‘start of RRT vintage.’
When analyzing the association of LR with transplantation, children aged <2 years were excluded (n=188) as their age-related weight was likely to make them unsuitable for early transplantation. Children commencing RRT after December 31, 2011 were also excluded (n=83) to allow a minimum follow-up time of ≥1 year at the census date, December 31, 2012.
Statistical Analyses
Continuous data were analyzed and compared between groups (ER versus LR) using appropriate tests. We defined a center as large if their incident RRT population was >30 per 5-year period, as per a recent report of UKRR (24). A multivariable logistic regression model was developed to identify association variables with the probability of being LR, including age at first presentation, gender, ethnicity, time period at start of RRT, PRD, and center.
Survival analysis (as time to transplantation) was performed using Kaplan–Meier unadjusted survival and Cox regression proportional hazards models. Patients were censored if aged ≥18 years, they transitioned to adult services, at death, or at the census date, whichever was earlier. Patients receiving a pre-emptive transplant were excluded from the Cox regression model to evaluate the association of LR with time to transplantation, after adjusting by age at RRT start, gender, ethnicity, vintage of RRT start, and PRD. As proportional hazard assumptions were not satisfied for the variable of interest (LR), a piecewise Cox model was fitted to calculate adjusted hazard ratios for different time durations. The same method was used in the patient survival analysis, which considered the time from start of RRT to death. For this analysis, we did not exclude patients starting RRT at age <2 years. Hazard ratios were used to describe the independent relationship of LR with patient survival after adjusting for confounders (age at start of RRT, gender, and treatment modality). All analyses were performed using SAS software version 9.3, and P values of <0.05 were accepted as significant.
Results
Of 1741 eligible children, 7.9% (n=138) were excluded because of missing data for date first seen by a nephrologist. Of the remaining 1603 patients, 25.5% (n=408) of children starting RRT were identified as receiving a LR to pediatric renal services, of whom 54% (219 of 408) of children with LR commenced dialysis in <7 days and a further 19% (78 of 408) children in 7 to <30 days following initial presentation. The patient characteristics at baseline are shown in Table 1, with clinical characteristics by LR subgroups (<7, 7 to <30, and 30 to <90 days) in Supplemental Table 1. Overall, 25% of children starting RRT in the United Kingdom receive a LR to pediatric renal services, with little change observed over the past two decades.
Table 1.
Patient characteristics at baseline of all children and their distribution by late referral and early referral
| Variable | All Children | Late Referrals | Early Referrals | P Value |
|---|---|---|---|---|
| Total, n (%) | 1603 | 408 (25.5) | 1195 (74.5) | |
| Age at presentation, median (IQR), yr | 4.1 (0.3–10.1) | 11.0 (5.1–13.7) | 2.1 (0.1–7.6) | <0.001 |
| Age at presentation, n (%), yr | <0.001 | |||
| 0–<1 | 522 (32.6) | 37 (9.1) | 485 (40.6) | |
| 1–1.9 | 130 (8.1) | 27 (6.6) | 103 (8.6) | |
| 2–3.9 | 140 (8.7) | 24 (5.9) | 116 (9.7) | |
| 4–7.9 | 265 (16.5) | 50 (12.3) | 215 (18.0) | |
| 8–11.9 | 281 (17.5) | 95 (23.3) | 186 (15.6) | |
| 12–16 | 265 (16.5) | 175 (42.9) | 90 (7.5) | |
| Age at start of RRT, median (IQR) | 10.0 (5.1–13.4) | 11.1 (5.1–13.7) | 9.6 (5.0–13.2) | 0.06 |
| Age at start of RRT, n (%) | <0.001 | |||
| 3 mo–1.9 yr | 188 (11.7) | 62 (15.2) | 126 (10.5) | |
| 2–3.9 yr | 152 (9.5) | 25 (6.1) | 127 (10.6) | |
| 4–7.9 yr | 272 (17.0) | 51 (12.5) | 221 (18.5) | |
| 8–11.9 yr | 388 (24.2) | 94 (23) | 294 (24.6) | |
| 12–16 yr | 603 (37.6) | 176 (43.1) | 427 (35.7) | |
| Gender, n (%) | <0.001 | |||
| Female | 678 (42.3) | 231 (56.6) | 447 (37.4) | |
| Male | 925 (57.7) | 177 (43.4) | 748 (62.6) | |
| Ethnicity,a n (%) | 0.04 | |||
| Black | 49 (3.1) | 19 (4.7) | 30 (2.5) | |
| Other | 72 (4.5) | 25 (6.1) | 47 (3.9) | |
| South Asian | 267 (16.7) | 64 (15.7) | 203 (17.1) | |
| White | 1209 (75.7) | 299 (73.5) | 910 (76.5) | |
| Time period at start of RRT, n (%) | 0.94 | |||
| 1996–2001 | 529 (33.0) | 136 (33.3) | 393 (32.9) | |
| 2002–2007 | 558 (34.8) | 139 (34.1) | 419 (35.1) | |
| 2008–2012 | 516 (32.2) | 133 (32.6) | 383 (32.1) | |
| Primary renal diagnosis,b n (%) | <0.001 | |||
| Congenital nephrotic syndrome | 94 (5.9) | 14 (3.5) | 80 (6.7) | |
| Drug nephrotoxicity | 20 (1.3) | 9 (2.2) | 11 (0.9) | |
| Glomerular disease | 340 (21.4) | 119 (29.5) | 221 (18.6) | |
| Malignancy and associated disease | 22 (1.4) | 9 (2.2) | 13 (1.1) | |
| Metabolic | 77 (4.8) | 10 (2.5) | 67 (5.6) | |
| Obstructive uropathy | 241 (15.1) | 16 (4) | 225 (18.9) | |
| Polycystic kidney disease | 45 (2.8) | 6 (1.5) | 39 (3.3) | |
| Reflux nephropathy | 93 (5.8) | 21 (5.2) | 72 (6.1) | |
| Renal dysplasia | 404 (25.4) | 72 (17.9) | 332 (27.9) | |
| Renovascular disease | 47 (3.0) | 14 (3.5) | 33 (2.8) | |
| Tubulo-interstitial diseases | 134 (8.4) | 60 (14.9) | 74 (6.2) | |
| Uncertain etiology | 75 (4.7) | 53 (13.2) | 22 (1.9) | |
| Center size, n (%) | 0.08 | |||
| Small | 290 (18.1) | 62 (15.2) | 228 (19.1) | |
| Large | 1313 (71.9) | 346 (74.8) | 967 (70.9) |
IQR, interquartile range.
n=6 missing ethnicity.
n=11 missing PRD.
Children with LR were more likely to be older at time of first presentation, female, and black. There were significant differences for PRD between children with LR and ER (P<0.001). No significant differences between LR and ER patients were noted across different pediatric centers, center sizes (large/small), time period at start of RRT, or dialysis modality at start of RRT.
Following adjusted logistic regression analysis (Table 2), the oldest children, girls, and those of “other” ethnic origin were more likely to receive a LR. In this study “other” ethnicity included those from Chinese, other Asian groups, or those with mixed race ethnic origin.
Table 2.
Adjusted odds ratios of being referred late to a pediatric renal center by patient-specific variables
| Variable | Odds Ratio | 95% LCL | 95% UCL | P Value |
|---|---|---|---|---|
| Age at presentation, yr | ||||
| 0–<1 | 0.21 | 0.13 | 0.34 | <0.001 |
| 1–1.9 | 0.61 | 0.36 | 1.04 | 0.07 |
| 2–3.9 | 0.41 | 0.24 | 0.71 | <0.001 |
| 4–7.9 | 0.45 | 0.29 | 0.68 | <0.001 |
| 8–11.9 | 1 | |||
| 12–16 | 3.70 | 2.53 | 5.39 | <0.001 |
| Gender | ||||
| Female | 1.70 | 1.30 | 2.24 | <0.001 |
| Male | 1 | |||
| Ethnicitya | ||||
| Black | 1.21 | 0.60 | 2.46 | 0.59 |
| Other | 1.91 | 1.04 | 3.52 | 0.04 |
| South Asian | 0.76 | 0.52 | 1.12 | 0.16 |
| White | 1 | |||
| Time period at start of RRT | ||||
| 1996–2001 | 0.96 | 0.69 | 1.34 | 0.80 |
| 2002–2007 | 0.93 | 0.67 | 1.30 | 0.67 |
| 2008–2012 | 1 | |||
| Primary renal diagnosisb | ||||
| Congenital nephrotic syndrome | 1.46 | 0.74 | 2.87 | 0.28 |
| Drug nephrotoxicity | 1.67 | 0.61 | 4.61 | 0.32 |
| Glomerular disease | 1.60 | 1.12 | 2.29 | 0.01 |
| Malignancy and associated disease | 4.11 | 1.57 | 10.72 | 0.004 |
| Metabolic | 0.75 | 0.35 | 1.58 | 0.45 |
| Obstructive uropathy | 0.53 | 0.29 | 0.97 | 0.04 |
| Polycystic kidney disease | 1.10 | 0.43 | 2.85 | 0.84 |
| Renovascular disease | 2.14 | 0.98 | 4.65 | 0.05 |
| Tubulo-interstitial diseases | 2.37 | 1.49 | 3.78 | <0.001 |
| Uncertain etiology | 5.75 | 3.10 | 10.65 | <0.001 |
| Renal dysplasia and/or reflux nephropathy | 1 |
LCL, lower confidence limit; UCL, upper confidence limit.
n=6 missing ethnicity.
n=11 missing PRD.
Children diagnosed with glomerular disease (odds ratio [OR], 1.6; 95% confidence interval [95% CI], 1.12 to 2.29), malignancy and associated diseases (OR, 4.11; 95% CI, 1.57 to 10.72), tubulo-interstitial diseases (OR, 2.37; 95% CI, 1.49 to 3.78), and those with ESRD due to an uncertain etiology (OR, 5.75; 95% CI, 3.1 to 10.65) were more likely to receive a LR compared with children with renal dysplasia and/or reflux nephropathy. In contrast, children with obstructive uropathy were more likely to receive an ER (OR, 0.53; 95% CI, 0.29 to 0.97). The start of RRT vintage had no influence on the probability of LR. There was no significant association of center size (small versus large) with LR (OR, 0.8; 95% CI, 0.6 to 1.2; P=0.33) (Supplemental Table 2). Analysis in a subgroup of selected PRDs including dysplasia, reflux nephropathy, obstructive uropathy, polycystic kidney disease, and uncertain etiology, confirmed similar findings (Supplemental Tables 3 and 4).
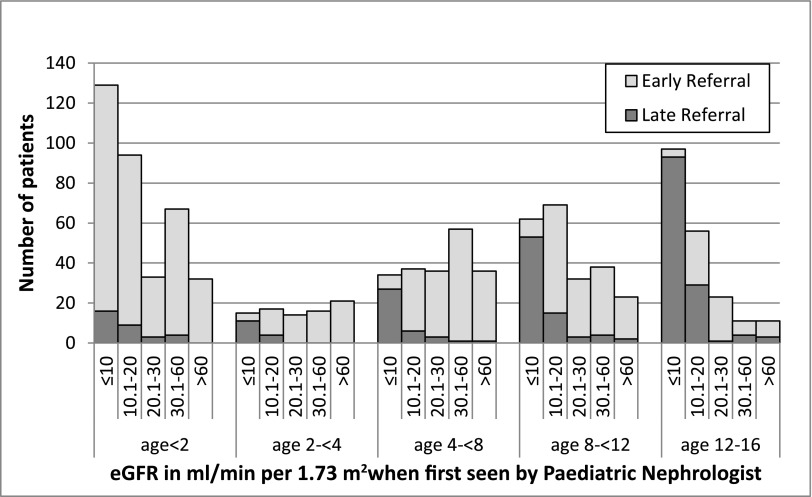
eGFR was available at the date first seen by a nephrologist in 66% (1060 of 1603) of the children. Distribution of patient characteristics and missing data were equally distributed across both LR and ER categories (data not shown). Figure 1 displays the distribution of eGFR with age at date first seen by a nephrologist. There were 337 (31.8%), 273 (25.8%), 138 (13%), 189 (17.8%), and 123 (11.6%) patients with eGFR of ≤10, 10.1–20, 20.1–30, 30.1–60, and >60 ml/min per 1.73 m2, respectively, at the date first seen by nephrologist. In those children with eGFR≤10 ml/min per 1.73 m2 when first seen, the proportion of LR in those aged >2 years versus those <2 years was 88% versus 12% (P<0.001).
Figure 1.
Distribution of eGFR in ml/min per 1.73 m2 with age in years at first review with pediatric nephrologist in children with early and late referral.
There were also significant differences in level of eGFR when first seen by nephrologist by PRD: in the majority with uncertain etiology, eGFR was <10 ml/min per 1.73 m2 as opposed to the majority with congenital nephrotic syndrome, glomerular disease, and metabolic disease, in whom eGFR was >30 ml/min per 1.73 m2 (P<0.001). No differences in eGFR levels were apparent for gender, ethnicity, pediatric center, and start of RRT vintage.
Association with Transplantation
During the study period, 461 children (28.8%) underwent pre-emptive transplantation; as expected, none of the children with LR received pre-emptive transplantation. Significant differences in transplantation rates continued to be observed in children with LR up to 1 year following commencement of RRT (Table 3). Of those transplanted, 34.9% were from living donors, with no significant association between donor type and referral group (33.3% and 35.3% living donor-related in LR and ER respectively, P=0.55).
Table 3.
Adjusteda hazard ratios of being transplanted,b by the time from start of RRT, if referred early as compared with late
| Time from start of RRT, months | Hazard Ratio | 95% Confidence Interval of Hazard Ratio |
|---|---|---|
| 0–<6 | 6.2 | 3.4 to 11.4 |
| 6–12 | 1.7 | 1.2 to 2.4 |
| >12 | 1.03 | 0.84 to 1.25 |
Adjusted by age at RRT start, gender, ethnicity, vintage of RRT start, and PRD.
This analysis excludes patients pre-emptively transplanted.
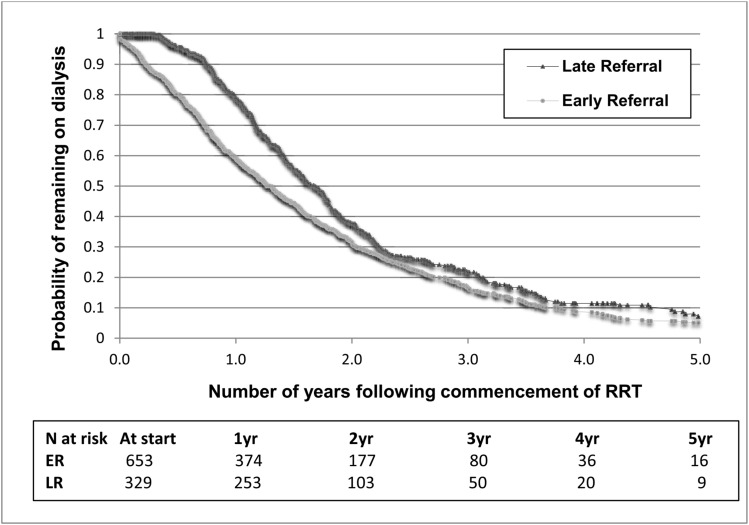
At 1 year after starting RRT, 61% of ERs had received a graft, while only 21% of LRs had received a graft. After 1 year there were no significant differences between ER and LR groups (Figure 2). The log-rank test for the Kaplan–Meier analyses was P<0.001.
Figure 2.
Kaplan–Meier estimate of time to transplantation, by timing of referral in children with RRT. Children that received pre-emptive transplants (all ER) and children aged <2 years at RRT start were excluded from this analysis. The log-rank test for the Kaplan–Meier analyses was P<0.001. ER, early referral; LR, late referral.
Association with Survival
The median (interquartile range) follow-up time was 4.8 years (2.9–7.6 years). During this time, there were 55 deaths, contributing to a survival probability at 5 years of 95.7% in LRs and 96.7% in ERs. Following adjustment in a Cox regression analysis, no statistically significant difference in survival was noted in the LR group compared with the ER group (hazard ratio, 1.30; 95% CI, 0.7 to 2.3; P=0.40).
Discussion
This national study of children commencing RRT highlights that LR is a significant issue affecting children with CKD. Overall, 25% of children commenced RRT <90 days following their first review with a pediatric nephrologist, 75% of whom commenced RRT in <30 days; there was little change in trends over the past two decades across the United Kingdom. Children with LR continued to display significantly lower rates of transplantation up to 1 year following commencement of dialysis. We observed no significant survival disadvantage in those with LR during childhood.
To define LR in children, both time-interval–based definitions and those using level of renal dysfunction when first seen by a nephrologist have been reported (19–21). Our study findings are comparable to other pediatric reports at 21%–35% (19,25), although these studies have defined LR using a shorter time interval at ≤1 month between initial presentation and commencement of RRT. It is somewhat disappointing to note that rates of LR in the United Kingdom have not changed significantly over the last two decades, despite significant investment in health care during this time and increasing evidence supporting ER of children with CKD to pediatric nephrology centers. Children with LR are unlikely to benefit from current management strategies that aim to delay progression to ESRD (26), increase rates of pre-emptive transplantation (21), and reduce medical and psychologic complications of CKD in childhood (26–29). Although incident rates of ESRD in children in the United Kingdom remain stable at 8–10 per million age-related population over this time period (30), one would anticipate that an improvement in diagnostic capabilities would have resulted in earlier diagnosis, prompting earlier referral.
In a single-center report by Kennedy et al. from Sydney, Australia, LR was defined as an eGFR<30 ml/min per 1.73 m2 at first presentation to a nephrologist. LR in children aged >1 year was reported as 55% (22 of 40) (20). In a subanalysis, they defined LR similar to our study here, and reported LR in 30% (14 of 47) of children. Rates of LR were about 30% when using both definitions, i.e., children with GFR<30 ml/min per 1.73 m2 at the time of first review to nephrologist and who commenced RRT within 90 days (20). Our study findings were comparable when using both eGFR at presentation and a time-interval–based definition of <90 days before commencing RRT; the number of children was comparable at 26%
A recent Europe-wide renal registry study (25), which includes some data from the United Kingdom, reported LR using eGFR at presentation based definitions and observed that 60% of patients had an eGFR<20 ml/min per 1.73 m2 when first seen by nephrologist. As expected, our study findings were similar, with 57.6% of patients with eGFR<20 ml/min per 1.73 m2 at first review. These data do not represent the eGFR at presentation in all children presenting with CKD to a nephrologist, but only those who commenced RRT during the study period; this is likely the explanation for the considerably lower eGFR overall at first nephrology review in this and other similar cohorts (25).
We observed that the oldest (≥12 years) and youngest (<2 years) cohorts had between 60%–80% of children present with eGFR<20 ml/min per 1.73 m2 when first seen by a nephrologist. Despite this, in those aged <2 years the rates of LR were significantly lower than older peers, reflecting the physiologic improvement in GFR over the first few years of life and perhaps the difficulties in providing RRT in the youngest children.
The differences by PRD between LR and ER patients is not surprising as children with certain conditions, like obstructive uropathy, are more likely to be diagnosed prenatally and thus referred earlier, especially when compared with those with GN. Additional causes for LR in those with GN may include disease-specific factors: the progression may be rapid and nonresponsive to treatment, leading to early onset of ESRD despite being referred soon after onset of symptoms. Children with tubulo-interstitial diseases were also observed to have high rates of LR, in keeping with their known nonspecific or lack of symptoms, frequently a lack of findings on urine examination, and slow progression through childhood. The six-fold increase in risk of LR with uncertain etiology, highlights in part the difficulties of diagnosing renal disease in children and the probable insidious presentation of this PRD in this cohort.
As expected, none of the LR patients in this study received pre-emptive transplantation. Thus, all patients with LR were disadvantaged by exposure to dialysis-related morbidities, both physical and psychosocial. Significant differences in rates of transplantation remained evident between LR and ER patients until 1 year following commencement of dialysis, and probably reflects the time taken to prepare the child and their family for kidney transplantation. Deceased donor allografts in the United Kingdom are allocated as per nationally agreed guidelines from the National Health Service Blood and Transplant (NHSBT). These are available at the NHSBT website or can be directly accessed at http://www.odt.nhs.uk/pdf/kidney_selection_policy.pdf. Interestingly, we observed no adverse association of LR with patient survival during the study period. This may have been because the duration of follow-up was limited to the time period during childhood only. These findings may also reflect the overall quality of care received by children on RRT in the United Kingdom, and these may not be extendable to other RRT populations, particularly in resource-limited settings.
We believe our data have implications for childhood CKD populations worldwide, in particular, regarding the knowledge of any inherent factors with children that limit the early detection of impaired renal function leading to LR. These may include nonspecific symptoms such as tiredness and lethargy seen with CKD, the insidious progression of CKD, and the challenges in the accurate measurement of BP and in the collection of urine specimens for testing in children. Our data highlights the need for an urgent improvement in our understanding of these and other contributing factors, as it is likely that in a proportion of children with LR, a delay in the diagnosis of CKD remains possible. Early diagnosis and referral of CKD to nephrologists will undoubtedly benefit children and their families by allowing access to specialist multidisciplinary team-based renal care, establishing of PRD and its specific therapy where indicated, for optimization of BP control, and improved management of anemia and proteinuria. These together are likely to help delay the progression of renal dysfunction, need for early RRT (26), and may lead to an improvement in rates of pre-emptive transplantation. Understanding the factors contributing to LR is also important to help develop robust algorithms in primary and secondary care to increase awareness among health professionals regarding kidney function and kidney injury in children. In the United Kingdom adult RRT population, late presentation has steadily declined from 23.9% in 2003 to 18.4% in 2006 (1). This may be a consequence of national efforts to raise awareness among non-nephrologists using national guidelines (31), health care quality initiatives, and introduction of eGFR reporting, as has also been reported recently outside the United Kingdom (32). Further research is still needed in children with CKD to understand the benefit of earlier referral on long-term clinical outcomes including survival, transplantation, and quality of life.
Our study is not without limitations and these include lack of information on socioeconomic status and comorbidity data, as these could be a cause of inherent residual confounding. We do not have uniform information regarding prenatal diagnosis in the pediatric UKRR database and referral patterns of prenatally suspected renal disease to a pediatric nephrologist; this might have resulted in a slight overestimation of LR and may have also prevented the study from detecting a change in LR over time. Despite this, these data represent the most thorough analyses of LR in a United Kingdom-wide large pediatric cohort. Similar to other reports on LR in children and adults, we are unable to identify specific factors that may contribute to LR in our cohort as these data are not collected routinely as part of the annual UKRR dataset. Missing data, especially for GFR at referral, and the availability of only one value of GFR before commencement of RRT in most cases also limits our findings regarding the rate of decline in renal function in children with LR. We also do not have any detailed information regarding preexisting renal disease and, if present, its duration. These data are critical to accurately define cases as LRs as it is likely that some cases with glomerular disease, malignancy, and tubulo-interstitial disease had a rapid progression of renal dysfunction. Data reported to the UKRR is often collected at the end of the year and not at the time of initial presentation, and is thus retrospective at point of collection. Finally, the patients in this registry-based analyses do not include children that did not survive dialysis for >90 days despite commencement of RRT for irreversible renal failure, as data for these children are not collected as part of the UKRR dataset.
In conclusion, a quarter of children starting RRT in the United Kingdom are late in presenting to pediatric renal services, with little change observed in trends over almost two decades. Late presentation of children to a nephrologist has a significant impact on the child and their subsequent renal outcomes, with children referred late often needing dialysis rapidly, being unable to benefit from pre-emptive transplantation and requiring longer periods of dialysis while awaiting kidney transplantation. Further data are needed to accurately ascertain children with preexisting renal disease who subsequently present as LR. There is an urgent need to understand causes of avoidable LR during childhood and a need for strategies to improve kidney awareness more widely among health care professionals looking after children.
Disclosures
None.
Supplementary Material
Acknowledgments
Author M.D.S. acknowledges financial support from the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's & St, Thomas' National Health Service Foundation Trust in partnership with King's College London and King’s College Hospital National Health Service Foundation Trust.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08190815/-/DCSupplemental.
References
- 1.Gilg J, Pruthi R, Fogarty D: UK Renal Registry 17th Annual Report: Chapter 1 UK Renal Replacement Therapy Incidence in 2013: National and Centre-specific Analyses. Nephron 129[Suppl 1]: 1–29, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Cass A, Cunningham J, Arnold PC, Snelling P, Wang Z, Hoy W: Delayed referral to a nephrologist: outcomes among patients who survive at least one year on dialysis. Med J Aust 177: 135–138, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Curtis BM, Barret BJ, Jindal K, Djurdjev O, Levin A, Barre P, Bernstein K, Blake P, Carlisle E, Cartier P, Clase C, Culleton B, Deziel C, Donnelly S, Ethier J, Fine A, Ganz G, Goldstein M, Kappel J, Karr G, Langlois S, Mendelssohn D, Muirhead N, Murphy B, Pylpchuk G, Toffelmire E; CREDA (Canadian Renal Disease Alliance) : Canadian survey of clinical status at dialysis initiation 1998-1999: A multicenter prospective survey. Clin Nephrol 58: 282–288, 2002 [PubMed] [Google Scholar]
- 4.Stoves J, Bartlett CN, Newstead CG: Specialist follow up of patients before end stage renal failure and its relationship to survival on dialysis. Postgrad Med J 77: 586–588, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkelmayer WC, Owen WF Jr, Levin R, Avorn J: A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J Am Soc Nephrol 14: 486–492, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Smart NA, Titus TT: Outcomes of early versus late nephrology referral in chronic kidney disease: a systematic review. Am J Med 124: 1073–80.e2, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Jungers P, Joly D, Nguyen-Khoa T, Mothu N, Bassilios N, Grünfeld JP: [Continued late referral of patients with chronic kidney disease. Causes, consequences, and approaches to improvement]. Presse Med 35: 17–22, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Van Biesen W, Wiedemann M, Lameire N: End-stage renal disease treatment: a European perspective. J Am Soc Nephrol 9[Suppl]: S55–S62, 1998 [PubMed] [Google Scholar]
- 9.Stack AG: Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am J Kidney Dis 41: 310–318, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Roderick P, Jones C, Drey N, Blakeley S, Webster P, Goddard J, Garland S, Bourton L, Mason J, Tomson C: Late referral for end-stage renal disease: a region-wide survey in the south west of England. Nephrol Dial Transplant 17: 1252–1259, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Wauters JP, Lameire N, Davison A, Ritz E: Why patients with progressing kidney disease are referred late to the nephrologist: on causes and proposals for improvement. Nephrol Dial Transplant 20: 490–496, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Huisman RM: The deadly risk of late referral. Nephrol Dial Transplant 19: 2175–2180, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Lameire N, Wauters JP, Teruel JL, Van Biesen W, Vanholder R: An update on the referral pattern of patients with end-stage renal disease. Kidney Int Suppl 61: 27–34, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Levin A: Consequences of late referral on patient outcomes. Nephrol Dial Transplant 15[Suppl 3]: 8–13, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Gøransson LG, Bergrem H: Consequences of late referral of patients with end-stage renal disease. J Intern Med 250: 154–159, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Hommel K, Madsen M, Kamper AL: The importance of early referral for the treatment of chronic kidney disease: a Danish nationwide cohort study. BMC Nephrol 13: 108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pradel FG, Jain R, Mullins CD, Vassalotti JA, Bartlett ST: A survey of nephrologists’ views on preemptive transplantation. Clin J Am Soc Nephrol 3: 1837–1845, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seikaly MG, Salhab N, Browne R: Patterns and time of initiation of dialysis in US children. Pediatr Nephrol 20: 982–988, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Jander A, Nowicki M, Tkaczyk M, Roszkowska-Blaim M, Jarmoliński T, Marczak E, Pałuba E, Pietrzyk JA, Siteń G, Stankiewicz R, Szprynger K, Zajaczkowska M, Zachwieja J, Zoch-Zwierz W, Zwolińska D: Does a late referral to a nephrologist constitute a problem in children starting renal replacement therapy in Poland?--a nationwide study. Nephrol Dial Transplant 21: 957–961, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kennedy SE, Bailey R, Kainer G: Causes and outcome of late referral of children who develop end-stage kidney disease. J Paediatr Child Health 48: 253–258, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Boehm M, Winkelmayer WC, Arbeiter K, Mueller T, Aufricht C: Late referral to paediatric renal failure service impairs access to pre-emptive kidney transplantation in children. Arch Dis Child 95: 634–638, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, Balk E, Lau J, Levin A, Kausz AT, Eknoyan G, Levey AS; National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative : National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics 111: 1416–1421, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruthi R, Hamilton AJ, O’Brien C, Casula A, Braddon F, Inward C, Lewis M, Maxwell H, Stojanovic J, Tse Y, Sinha MD: UK Renal Registry 17th Annual Report: Chapter 4 Demography of the UK Paediatric Renal Replacement Therapy Population in 2013. Nephron 129[Suppl 1]: 87–98, 2015 [DOI] [PubMed] [Google Scholar]
- 25.van Stralen KJ, Tizard EJ, Jager KJ, Schaefer F, Vondrak K, Groothoff JW, Podracká L, Holmberg C, Jankauskiené A, Lewis MA, van Damme-Lombaerts R, Mota C, Niaudet P, Novljan G, Peco-Antic A, Sahpazova E, Toots U, Verrina E: Determinants of eGFR at start of renal replacement therapy in paediatric patients. Nephrol Dial Transplant 25: 3325–3332, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F; ESCAPE Trial Group : Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Gerson A, Hwang W, Fiorenza J, Barth K, Kaskel F, Weiss L, Zelikovsky N, Fivush B, Furth S: Anemia and health-related quality of life in adolescents with chronic kidney disease. Am J Kidney Dis 44: 1017–1023, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Gerson AC, Butler R, Moxey-Mims M, Wentz A, Shinnar S, Lande MB, Mendley SR, Warady BA, Furth SL, Hooper SR: Neurocognitive outcomes in children with chronic kidney disease: Current findings and contemporary endeavors. Ment Retard Dev Disabil Res Rev 12: 208–215, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Duquette PJ, Hooper SR, Wetherington CE, Icard PF, Gipson DS: Brief report: intellectual and academic functioning in pediatric chronic kidney disease. J Pediatr Psychol 32: 1011–1017, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Pruthi R, O’Brien C, Casula A, Braddon F, Lewis M, Maxwell H, Stojanovic J, Tse Y, Inward C, Sinha MD: UK Renal Registry 16th annual report: chapter 7 demography of the UK paediatric renal replacement therapy population in 2012. Nephron Clin Pract 125: 127–138, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Guideline Development Committee, Joint Specialty Committee on Renal Disease of the Royal College of Physicians of London and the Renal Association: Identification, management and referral of adults with chronic kidney disease: concise guidelines. Clin Med(Lond) 5: 635–642, 2005 [DOI] [PMC free article] [PubMed]
- 32.Foote C, Clayton PA, Johnson DW, Jardine M, Snelling P, Cass A: Impact of estimated GFR reporting on late referral rates and practice patterns for end-stage kidney disease patients: a multilevel logistic regression analysis using the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). Am J Kidney Dis 64: 359–366, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.