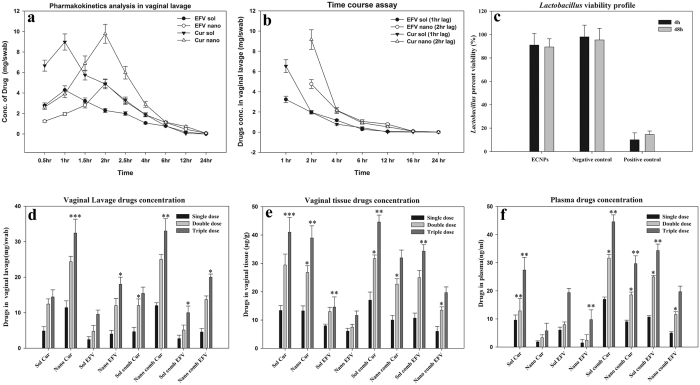
Figure 4.
(a) Pharmacokinetics study: 20 mg of sol curcumin plus 10 mg of sol EFV and an equivalent amount of ECNPs were applied as single dose in vagina for indicated time points. (b) Time course experiment: same drug doses have been topically applied at a lag period of 1 h (for sol EFV + Cur) and 2 hr (for ECNPs). Lavages were collected after these time points and curcumin and efavirenz concentration were calculated separately. Abbreviation: EFV sol and Cur sol – concentration of EFV and Cur when delivered via Sol (EFV + Cur). EFV nano and Cur nano – concentration of EFV and Cur when delivered via ECNPs. (c) Viability percentage of Lactobacillus cripatus when treated with ECNP, at 4 h and 48 h, media without ECNPs and 1% triton X served as negative and positive control respectively. (d–f) Dose-dependent study of drugs in single form or combination form either in soluble EFV + Cur or ECNPs. d, e and f represents the concentration of drugs in vaginal lavage, vaginal tissue and plasma respectively. Sample data were recorded as Mean ± SD, n = 3 and value of significance expressed as ***P < 0.0005, **P < 0.005, *P < 0.05. Abbreviation: EFV-comb-sol & Cur-comb-sol: - efavirenz and curcumin concentration delivered as soluble combination. EFV-comb-nano & Cur-comb-nano: - efavirenz and curcumin concentration delivered via ECNPs. EFV-sol: Soluble EFV. Cur-sol: Soluble Cur. EFV-nano: EFV released form ENP. Cur-nano: - Cur release form CNP.