Figure 5. Ubp2 removes long K63-linked ubiquitin chains from poly-ubiquitylated PCNA in vitro.
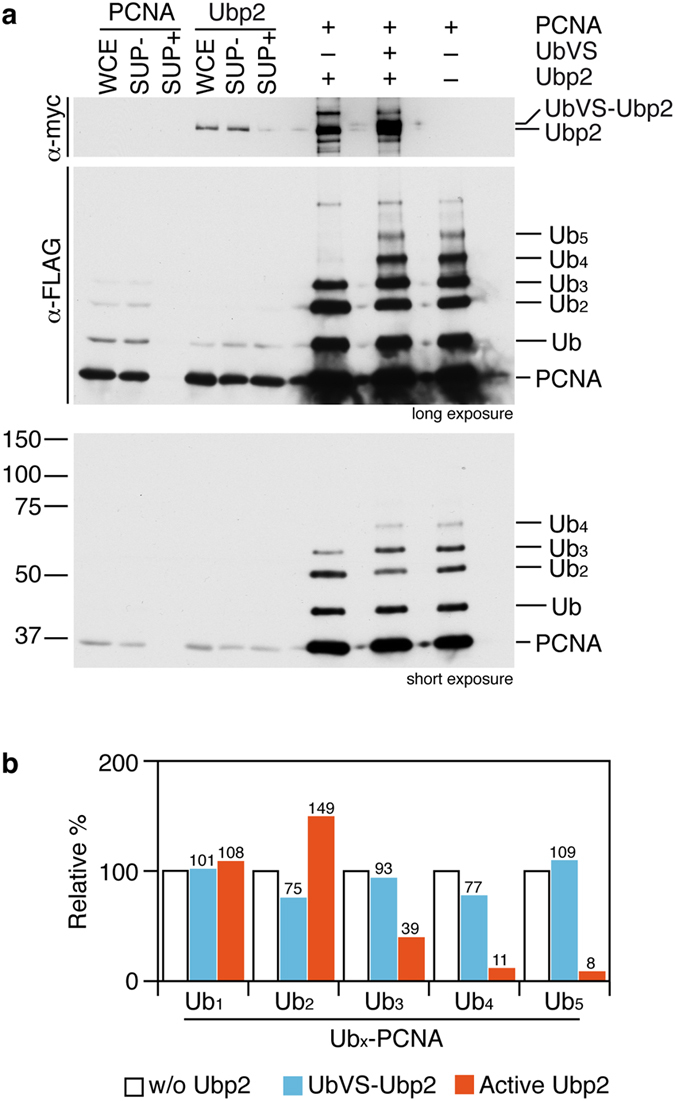
(a) Ubp2-myc deubiquitylates in vitro polyubiquitylated PCNA. Mono-, di-, and poly-ubiquitylated (ub3-to-ub6) PCNA was obtained by immunoprecipitation with anti-FLAG antibody from a Δ ubp2 ubp12-NES ubp15-NES Δ ubp16 pcn1-FLAG strain (synchronized in early S-phase; 3 hours 20 mM HU). Immunoprecipitated samples were divided in three; two of the aliquots were incubated with immunoprecipitated Ubp2-myc in the absence or in the presence of UbVS to inhibit the protease activity of Ubp2 (as described in the Methods section). The third aliquot served as a reference sample of the immunoprecipitated PCNA. PCNA deubiquitylation was detected by the distinctive SDS-PAGE gel mobility of the different PCNA forms. Whole cell extracts (WCE), depleted supernatants (SUP+ ), and non-depleted supernatants (SUP− ) from PCNA-FLAG and Ubp2-myc strains are also shown. (b) Signals of the different PCNA forms in the deubiquitylation reactions and controls resolved in the Western blots were quantified and plotted.
