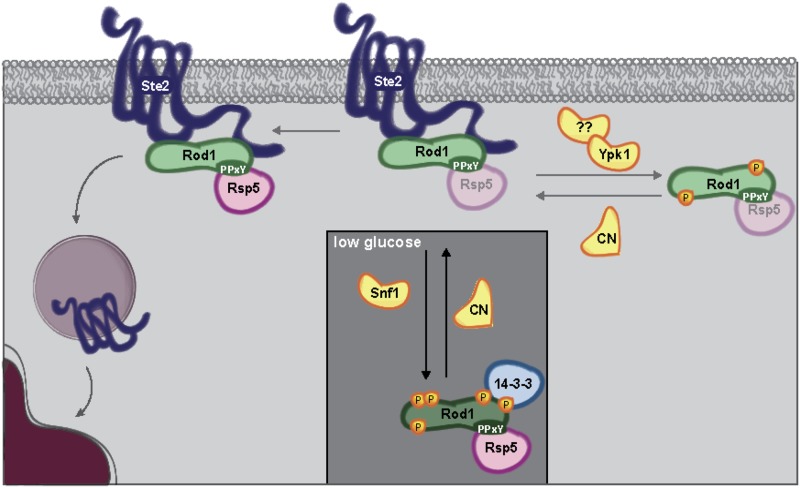
Figure 6.
Phospho-regulation of Rod1 function in mating pathway desensitization. Under normal growth conditions, Rod1 is phosphorylated at multiple sites that do not prevent its interaction with Rsp5, but do prevent its productive association with Ste2. Conditions that activate the phosphoprotein phosphatase calcineurin, or that diminish the activities of the protein kinases Snf1 and Ypk1, or both, permit Rod1-receptor association, promoting the Rsp5-dependent ubiquitinylation and clathrin-mediated endocytosis of Ste2. When phosphorylation of Rod1 at its Snf1 and Ypk1 sites is blocked, the only way it can be removed from the receptor is via its own Rsp5- and ubiquitin-dependent and proteasome-mediated destruction. When Rod1 cannot be phosphorylated at its Snf1 and Ypk1 sites and its V/PPxY are mutated (preventing Rsp5 recruitment), Rod1 remains bound to Ste2, blocking the ability of the receptor to stimulate its cognate G-protein and thereby potently squelching mating pheromone-evoked growth arrest.