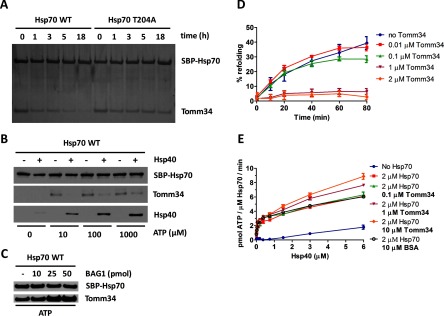
Fig. 3.
Tomm34 binding to Hsp70·ATP is dependent on ATP hydrolysis rate modulated by Hsp40 and Bag-1 co-chaperones and influences chaperone activity. A, SBP-Hsp70·ATP/Tomm34 complexes preformed in the presence of ATP (0.2 mm) were washed in nucleotide-free buffer and incubated at 4 °C for the indicated times before elution by biotin. The level of eluted proteins was analyzed by SDS-PAGE and silver staining. B, equimolar amounts (25 pmol) of SBP-Hsp70, Tomm34, and Hsp40 (as indicated) were mixed in increasing concentrations of ATP, eluted by biotin, and analyzed by Western blotting. C, equimolar amounts (25 pmol) of SBP-Hsp70 and Tomm34 were mixed with increasing amounts of Bag-1 in the presence of ATP (0.2 mm). Eluted proteins were analyzed by Western blotting. D, firefly luciferase was chemically denatured, mixed with Hsp70 (1 μm), Hsp40 (2 μm), ATP (1 mm), and varying Tomm34 concentrations, and recovered luminescence was measured. Negative controls described under “Experimental Procedures” did not exhibit luciferase activity and are not shown. E, ATPase activity of Hsp70 (2 μm) was tested at various Hsp40 and Tomm34 concentrations in malachite green assay. Refolding and ATPase experiments were performed in independent triplicates. Error bars represent S.E.