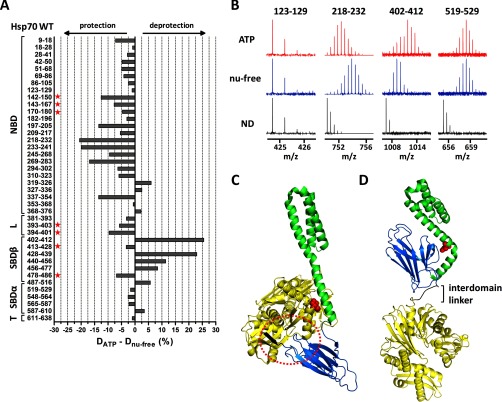
Fig. 4.
ATP-induced allosteric domain docking of Hsp70. A, deuteration level differences of Hsp70 peptides in ATP-bound and nucleotide-free state after 1 h of incubation in deuterated buffer. Numbers at left indicate the Hsp70 peptide fragments; schematic representation at left shows Hsp70 domain constitution; L, interdomain linker; T, C-terminal tail. B, original mass spectra of representative peptide fragments of Hsp70 incubated in non-deuterated buffer (ND) or for 1 h in deuterated buffer in the presence/absence of ATP. C and D, crystal structures of Hsp70 bacterial homologue DnaK in ATP-bound state (C, PDB code 4jne) and in nucleotide-free/ADP state (D, PDB code 2kho). NBD, interdomain linker, SBDβ, and SBDα are shown in yellow, black, marine, and green, respectively. Encircled region (C) shows tight association of IA subdomain of NBD, linker, and SBD loops L2,3/L6,7 (peptides indicated with a star in A) when SBDβ is docked onto NBD (compare with I164D mutant, Fig. 5A). Residues I160 and D526 (human numbering I164 and D529) are highlighted in red in ATP/ADP structures, respectively. The images were created in PyMOL.