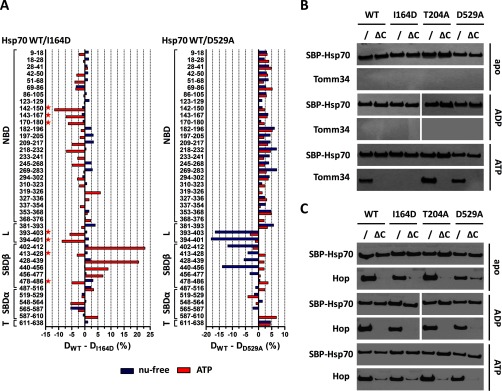
Fig. 5.
Hsp70 I164D lacks the ability to induce NBD-SBDβ docking upon ATP binding, whereas D529A mutant exhibits destabilized SBDβ subdomain/linker conformation in nucleotide-free state. In contrast to Hsp70/Hop binding, the efficient Hsp70/Tomm34 interaction is dependent on NBD-SBDβ docking. A, deuteration level differences of Hsp70 WT and I164D (or D529A) peptides in ATP-bound (red) or nucleotide-free state (blue) after 1 h of incubation in deuterated buffer. Peptides indicated with a star (left panel) highlight the impaired NBD-SBDβ domain docking of I164D mutant (compare with WT, Fig. 4C). B and C, purified SBP-tagged WT or mutant Hsp70s immobilized on streptavidin-agarose beads were incubated with Tomm34 (B) or Hop (C) in buffer containing 0.2 mm ATP, ADP, or no nucleotide. After washing, eluted proteins were analyzed by Western blotting. ΔC, deletion of PTIEEVD.