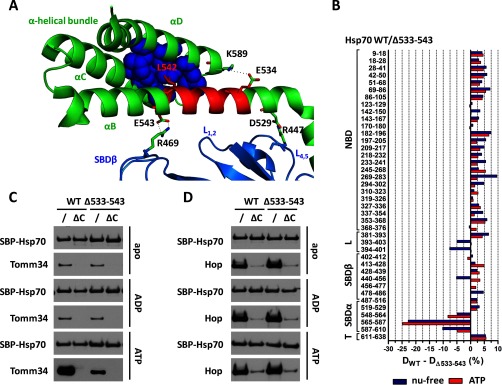
Fig. 8.
Deletion of 533–543 region severely decreased EEVD/ATP-dependent Hsp70/Tomm34 interaction without abrogating allosteric domain docking. However, the deletion has led to destabilization of the α-helical bundle. A, crystal structure of human SBD (PDB code 4po2). SBDβ, SBDα, and 533–543 regions are shown in marine, green, and red, respectively. The lid-SBDβ positioning ionic contacts (D529–R447 and E543–R469) and residues important for structural integrity of α-helical bundle by forming ionic contacts (E534–K589) and hydrophobic core (L542 shown in red, V577, W580, L581, F592, K595, and L599 shown as blue spheres) are indicated. B, deuteration level differences of Hsp70 WT and Δ533–543 peptides in ATP-bound (red) or nucleotide-free state (blue) after 1 h of incubation in deuterated buffer. C and D, purified SBP-Hsp70 WT or Δ533–543 immobilized on streptavidin-agarose beads were incubated with Tomm34 (C) or Hop (D) in buffer containing 0.2 mm ATP, ADP, or no nucleotide. Eluted proteins were analyzed by Western blotting. ΔC, deletion of PTIEEVD.