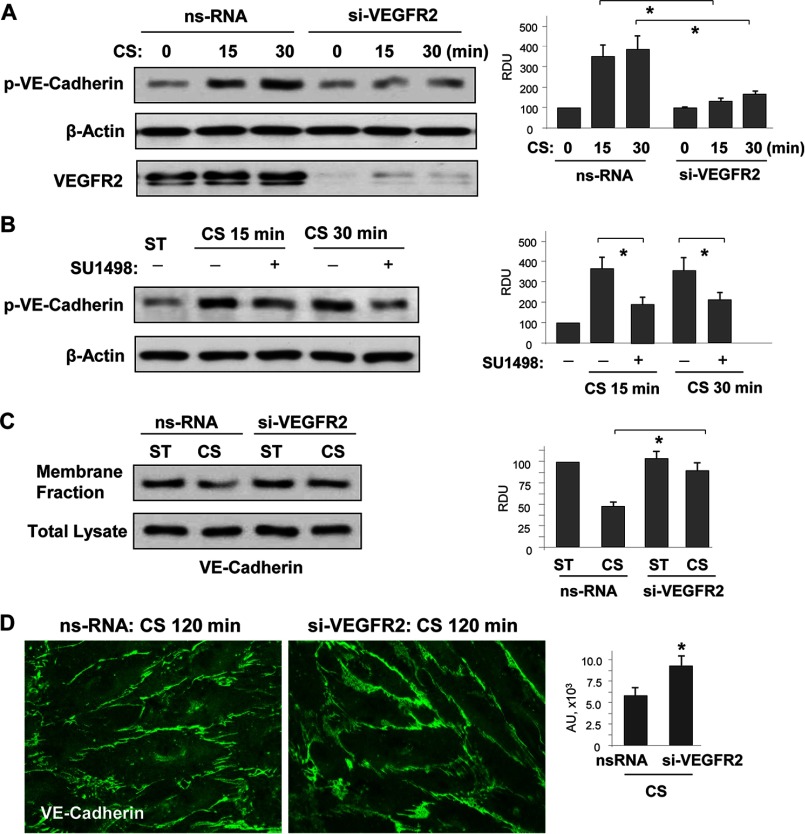
FIGURE 6.
18% CS-induced VEGFR2 activation mediated VE-cadherin phosphorylation, internalization, and disassembly of adherens junctions. Human pulmonary EC were transfected with VEGFR2-specific or nonspecific siRNA, followed by 18% CS stimulation for indicated periods of time. Control cells were left under static conditions. A, effect of VEGFR2 knockdown on CS-induced VE-cadherin tyrosine phosphorylation. siRNA-induced protein depletion was confirmed by Western blot. β-Actin was used as a normalization control. n = 3; *, p < 0.05 versus ns-RNA. B, effect of inhibition of VEGFR2 tyrosine kinase activity on CS-induced VE-cadherin tyrosine phosphorylation. The cells were treated with vehicle or 5 μm SU-1498 for 30 min prior to exposure to cyclic stretch. VE-cadherin phosphorylation was analyzed by Western blot with corresponding antibody. β-Actin was used as a normalization control. n = 3; *, p < 0.05 versus 18% CS alone. C, effect of VEGFR2 knockdown on 18% CS-induced VE-cadherin internalization was detected by Western blot analysis of protein content in the membrane faction and total lysates from static and stretched EC. n = 3; *, p < 0.05 versus ns-RNA. D, effect of VEGFR2 knockdown on 18% CS-induced adherence junction remodeling was evaluated by immunofluorescence staining of EC monolayers with VE-cadherin antibody. The bar graph represents quantitative analysis of VE-cadherin peripheral accumulation. The data are expressed as means ± S.D. of three independent experiments; *, p < 0.05 versus ns-RNA.