FIGURE 3.
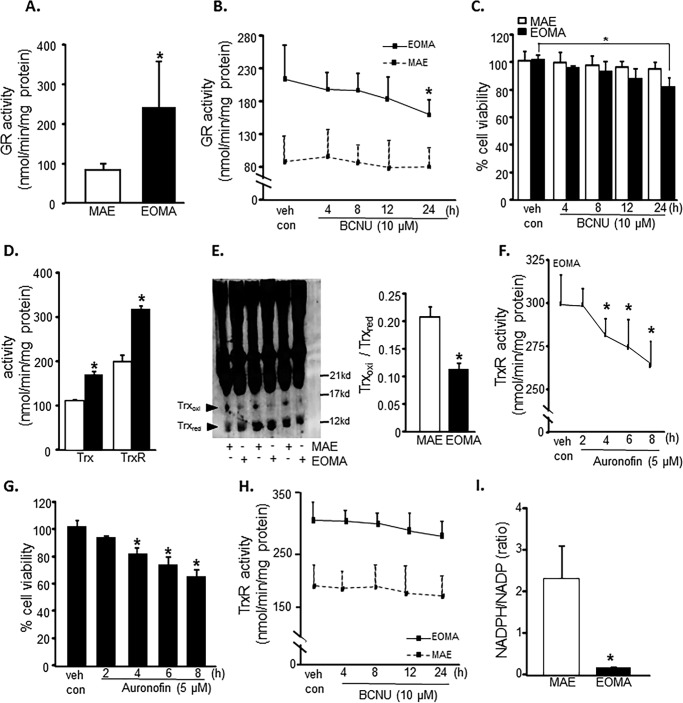
Elevated thiol reductase activity is necessary for EOMA cell survival. A, glutathione reductase (GR) activity as measured by NADPH oxidation showed significantly elevated levels of glutathione reductase in EOMA versus MAE cells. B, the glutathione reductase activity in EOMA cells was significantly reduced after a 24-h treatment with 10 μm BCNU, whereas MAE did not have loss of GR activity during the 24-h treatment period. C, LDH leakage toxicity assay for cell viability correlated with glutathione reductase inhibition data. D, thioredoxin (Trx) and thioredoxin reductase (TrxR) activities were measured using a single assay system (Cayman Chemical), and both were increased in EOMA cells compared with MAE cells. E, immunoblotting of thioredoxin after native gel (native PAGE 4–16% BisTris gel) electrophoresis showing the oxidation status of thioredoxin protein in both EOMA and MAE cells. The formation of intermolecular disulfide bridges as a result of oxidation could impede migration of oxidized thioredoxin in MAE compared with EOMA cells. Both cell lines have single bands observed at 14 kDa. The protein densitometry calculation signifies that the ratio of oxidized to reduced thioredoxin is higher in MAE than EOMA. F, treatment of EOMA cells with 5 μm auranofin demonstrated a decrease in TrxR activity over time, which correlated with the LDH toxicity data (G) indicating that EOMA cells are highly susceptible to loss of TrxR activity. H, because BCNU treatment is reported to inhibit TrxR activity (84), we measured the TrxR activity level in the presence of BCNU (10 μm), which shows no significant inhibition at 24 h of treatment of both cell lines. I, NADPH/NADP measurements in normally cultured EOMA and MAE cells were obtained using a single assay system (Sigma). Results are expressed as mean ± S.D. (error bars); *, p < 0.05.