TABLE 2.
Full list of interactions between Arg672 in WT MyHC-emb and the two Arg672 mutants
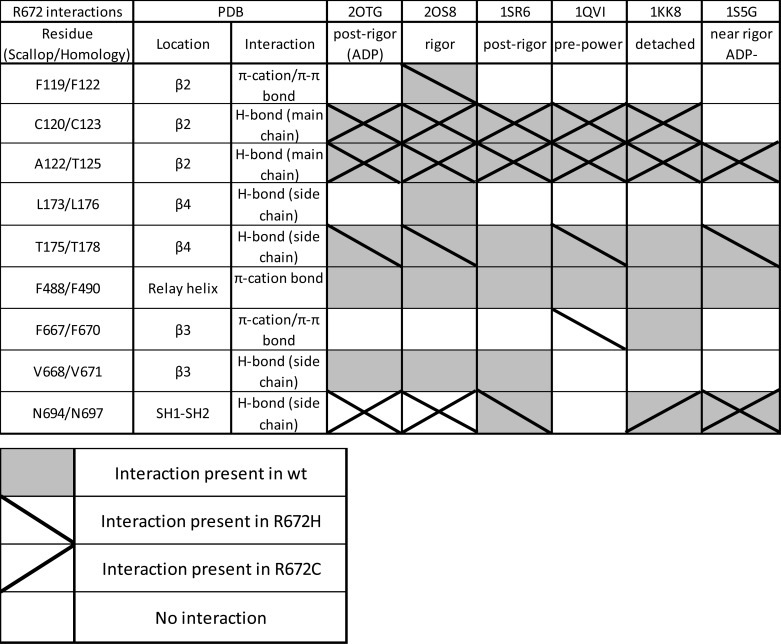
There are interactions between Arg672, on β3, and Cys123 and Thr125, on β2, of the central β-sheet in most conformations, the exception being the contact to Cys123 in the near rigor, ADP-bound state (PDB 1sS5gG). These interactions are predicted to be conserved in the R672C and R672H mutations. A π-cation bond that exists between Arg672 and Phe122 (β2) in the rigor state (2osOS8) is lost in both mutations, but a novel π-π bond is formed between R672H and Phe122 in the rigor state (20sOS8). The hydrogen bond between Arg672 and Leu176 (β1) is also lost in both mutations in the rigor state. Thr178 (on β4) hydrogen-bonds to Arg672 in each structure; however, this interaction is lost in the post-rigor (1srSR6) and the detached (1kkKK8) states for R672H and is lost completely for R672C. A second significant loss of interaction is the π-cation bond between Arg672 and Phe490 on the relay helix, which is non-existent in either mutation. A π-cation bond to Phe670 is lost for both mutations in the detached state; however, a π-π bond is formed in the pre-power state (1qviQVI). A hydrogen bond between Val671 is present in WT MyHC-emb in the rigor and post-rigor states (1osOS8, 2otgOTG, and 1srSR6) but lost in both mutations. The hydrogen bond between Asn697 on the SH1-SH2 domain is conserved in all three models in the near rigor ADP-bound state (1sS5Gg). However, this is lost in R672H in the detached state (1kkKK8) and in R672C in the post-rigor state (1srSR6). Interestingly, this interaction is formed in both mutants in the rigor (1kkKK8) and post-rigor (2OTGotg) states.