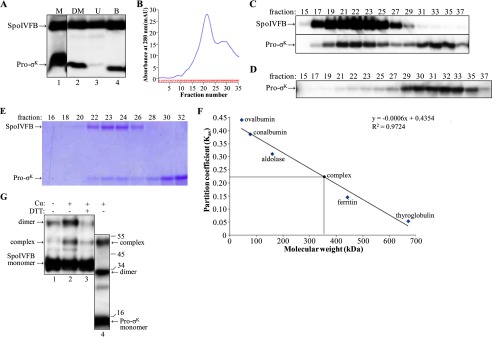
FIGURE 5.
Purification and characterization of the SpoIVFB-TEV-FLAG2 E44Q·Pro-σK(1–126)-His6 complex. A, cobalt affinity purification. E. coli bearing pYZ42 was grown in a fermentor and induced with IPTG to coexpress Pro-σK(1–126)-His6 and SpoIVFB-TEV-FLAG2 E44Q. The membrane (M) fraction was treated with DM to solubilize the complex followed by high-speed centrifugation and collection of a sample of the supernatant (DM), and the rest of the supernatant was subjected to cobalt affinity purification, resulting in unbound (U) and bound (B) samples, which were subjected to immunoblot analysis with anti-FLAG and anti-His antibodies. B, gel filtration chromatography. The bound fraction from the cobalt affinity purification was concentrated and applied to a size exclusion column. The absorbance at 280 nm was monitored as fractions were collected, generating the profile shown. C, immunoblot. Samples of fractions from the size exclusion column were subjected to immunoblot analysis with anti-FLAG (top) or anti-His (bottom) antibodies. D, purification of Pro-σK(1–126)-His6 alone. E. coli bearing pZR27 was grown in a fermentor and induced with IPTG to express Pro-σK(1–126)-His6. The membrane fraction was treated with DM, and the supernatant after high-speed centrifugation was subjected to cobalt affinity purification. The bound fraction was concentrated and applied to a size exclusion column, and samples of fractions were subjected to immunoblot analysis with anti-His antibodies. E, samples of fractions from a size exclusion column were subjected to SDS-PAGE as described for immunoblot analysis, but in this case gel electrophoresis was followed by Coomassie Blue staining of proteins. F, molecular weight estimate of the complex. The size exclusion column was calibrated with the indicated proteins. The partition coefficient was calculated as Kav = (elution volume − column void volume)/(geometric column volume − column void volume). The equation of the best fit line and the correlation coefficient are shown, as is the estimated molecular mass of the complex (360 kDa, which includes associated detergent) based on its Kav (0.22). G, disulfide cross-linking. Single-Cys versions of cytTM-SpoIVFB-FLAG2 E44C and Pro-σK(1–126)-His6 K23C were coexpressed in E. coli, and the complex was purified as described for A and B. Fractions 22–24 were pooled, and samples of the pooled fractions were treated with 1 mm Cu2+(phenanthroline)3 (Cu) for 10 min (+) or with 3 mm 2-phenanthroline for 10 min (−) as a control and then mixed with sample buffer with or without DTT as indicated and subjected to immunoblot analysis with antibodies against the FLAG tag on single-Cys cytTM-SpoIVFB-FLAG2 E44C (lanes 1–3) or against the His6 tag on single-Cys Pro-σK(1–126)-His6 K23C (lane 4). The immunoblot was cut between lanes 3 and 4 and realigned based on the migration of prestained marker proteins, for which sizes in kDa are indicated along the right side. Representative results from two biological replicates are shown.