Abstract
Background
In aortic stenosis (AS), symptoms and left ventricular (LV) dysfunction represent a later disease state, and objective parameters that identify incipient LV dysfunction are needed. We sought to determine prognostic utility of brain natriuretic peptide (BNP) and left ventricular global longitudinal strain (LV‐GLS) in patients with aortic valve area <1.3 cm2.
Methods and Results
Five‐hundred and thirty‐one patients between January 2007 and December 2008 with aortic valve area <1.3 cm2 (86% with aortic valve area ≤1.1 cm2) and left ventricular ejection fraction ≥50% who had BNP drawn ≤90 days from initial echo were included. Society of Thoracic Surgeons (STS) score and mortality were recorded. Mean STS score, glomerular filtration rate, and median BNP were 11±5, 73±35 mL/min per 1.73 m , and 141 (60–313) pg/mL, respectively; 78% were in New York Heart Association class ≥II. Mean LV‐stroke volume index (LV‐SVI) and LV‐GLS were 39±10 mL/m2 and −13.9±3%. At 4.7±2 years, 405 patients (76%) underwent aortic valve replacement; 161 died (30%). On multivariable survival analysis, age (hazard ratio [HR] 1.46), New York Heart Association class (HR 1.27), coronary artery disease (HR 1.72), decreasing glomerular filtration rate (HR 1.15), increasing BNP (HR 1.16), worsening LV‐GLS (HR 1.13) and aortic valve replacement (time dependent) (HR 0.34) predicted survival (all P<0.01). For mortality, the c‐statistic incrementally increased as follows (all P<0.01): STS score (0.60 [0.58–0.64]), STS score+BNP (0.67 [0.62–0.70]), and STS score+BNP+LV‐GLS (0.74 [0.68–0.78]).
Conclusions
In normal LVEF patients with significant aortic stenosis, BNP and LV‐GLS provide incremental (additive not duplicative) prognostic information over established predictors, suggesting that both play a synergistic role in defining outcomes.
Keywords: aortic stenosis, brain natriuretic peptide, global longitudinal strain
Subject Categories: Valvular Heart Disease, Echocardiography, Cardiovascular Surgery, Metabolism
Introduction
With an aging population, the prevalence of aortic stenosis (AS) is on the rise. AS presents as a continuum and patients are typically asymptomatic for a period of time, with onset of symptoms marking a key point in the natural history significantly impacting survival.1 Current guidelines recommend aortic valve replacement (AVR) for severe AS once symptoms occur or when there is ventricular systolic dysfunction.2 The presence of significant AS in the absence of symptoms and normal left ventricular ejection fraction (LVEF) presents a clinical dilemma. Increasingly, therefore, cardiologists are recognizing that various subtypes of AS with preserved LVEF have varying outcomes, when separated based on LV stroke volume index (LV‐SVI).3, 4, 5, 6 The clinician must balance the risk of AVR with risk of waiting for symptoms to develop. Waiting too long may have detrimental effects, as prior studies have linked severity of preoperative symptom status with worse postoperative outcome.7 It is increasingly being recognized that structural LV changes, in the setting of significant AS, may not always be reversible even after successful valve intervention and may impact long‐term survival, even in those with a normal LVEF. Additionally, many patients are relatively poor at identifying their symptomatic status due to functional limitation from aging or medical comorbidities. Thus, there is increasing interest in using sensitive markers of LV function, other than parameters derived from contractile function (LVEF or LV‐SVI), to determine outcomes in this population.8, 9, 10, 11, 12, 13, 14
Previous studies have established the usefulness of brain natriuretic peptide (BNP) in patients with AS.12, 14, 15, 16, 17, 18 These studies have found that BNP levels correlate with symptom‐free survival, New York Heart Association class, and survival.12, 16, 19, 20, 21 Left ventricular global longitudinal strain (LV‐GLS), measured using speckle tracking echocardiography, is a quantitative measure of early LV dysfunction, enabling assessment of longitudinally oriented subendocardial myocardial fibers, which are sensitive to ischemia and wall stress in AS patients. We sought to determine the incremental prognostic utility of BNP levels and LV‐GLS in a contemporary population of patients with significant AS and preserved LVEF.
Methods
Study Design
This was a retrospective observational cohort study of 531 patients who had an echocardiogram at our tertiary center between January 2007 and January 2008 documenting an aortic valve area (AVA) ≤1.3 cm2, LVEF ≥50%, without severe tricuspid/mitral valvular disease and serum BNP measured obtained close to the incident echocardiogram (>90% on the same day, all within 90 days) and without significant interval change in clinical status. We excluded patients with a limited life expectancy due to noncardiac causes (ie, terminal malignancy, stroke, and advanced lung disease) or death from noncardiac causes within 90 days of incident echocardiogram without having undergone AV surgery (n=15), LVEF <50% (n=94), and those with poor image quality for strain assessment (n=31).
Clinical Data
Clinical data were assembled from electronic medical records after appropriate Institutional Review Board approval. For BNP assay, all blood samples were collected into EDTA Vacutainer tubes. Specimens were immediately frozen and plasma was separated at −4°C. Plasma BNP (pg/mL) was determined by chemiluminescence immunoassay on site (Biosite Diagnostics, San Diego, CA). Cardiac procedures were as follows: (1) isolated AVR, (2) AVR and coronary artery bypass grafting, (3) AVR and ascending aorta repair or replacement +/− coronary artery bypass grafting, and (4) transcatheter AVR. The remainder were treated medically. Based on available preoperative data, Society of Thoracic Surgeons (STS) score was calculated. The decision for surgery was made by the individual treating cardiologists and cardiac surgeons at the time of clinical evaluation.
Outcomes Assessment
All‐cause mortality was considered to be the primary outcome. Death notification was confirmed by inspection of the death certificate or verified with a family member. In addition, we further categorized death as cardiac, noncardiac (eg, malignancy, cirrhosis of liver, primary pulmonary/neurologic etiology), or unknown. We also performed survival analysis for a secondary outcome of deaths, categorized as cardiac or unknown, but excluding documented noncardiac deaths (censoring these patients at the time of death). The duration of follow‐up ranged from initial echocardiogram to death or June 2013.
Echocardiographic Data
All patients underwent a comprehensive echocardiogram with commercially available instruments (Philips Medical Systems, General Electric, and Siemens Medical Solutions). Measurements were obtained according to recommendations and indexed to body surface area.22, 23, 24
For quantification of AS, LV outflow tract (LVOT) diameter was measured on parasternal long‐axis views. Pulsed‐wave and continuous‐wave Doppler was used to record velocities across LVOT and aortic valve (AV), respectively. LV‐SVI was measured using the following formula: LVOTVTI×LVOTarea/body surface area. A cutoff ≥35 mL/m2 was considered as preserved LV‐SVI.4, 23, 25, 26 AVA was calculated using the continuity equation and severe AS was defined as AVA ≤1 cm2 or mean AV gradient ≥40 mm Hg. Finally, valvuloarterial impedance (mm Hg·mL−1·m2), a measure of global LV afterload, was calculated as follows27: mean AV gradient+systolic blood pressure/LV‐SVI).
In all patients, LV‐GLS measurements were obtained from gray‐scale images recorded in apical 2, 3, and 4‐chamber views, using offline Velocity Vector Imaging (Syngo VVI; Siemens Medical Solutions, Mountain View, CA). The details of our protocol have been described previously.28 Measurements were made by an investigator blinded to all clinical information. LV‐GLS was not available to physicians at the time of surgical decision‐making.
Statistical Analysis
Continuous variables are expressed as mean (SD) and/or median and compared using analysis of variance (normal distribution) or Mann–Whitney test (non‐normal distribution). Categorical data are expressed as a percentage and compared using χ2. Association between continuous variables was tested using Spearman's correlation coefficient. To assess outcomes, multivariable Cox proportional hazards analysis was utilized. Relevant clinical and echocardiographic variables, known to be associated with outcomes in AS patients, were considered. AVR was included as a time‐dependent covariate in Cox analysis. For each patient undergoing AVR, the analysis time was modeled so that only the person‐time after AVR was included in the surgical group. The person‐time before AVR was included in the nonsurgical category. Hazard ratios with 95% CI were calculated. To ensure that proportional hazards assumption was not violated, graphical inspection of Schoenfield residuals plotted against time was performed. Additionally, survival curves for cumulative events as a function over time were obtained using Cox Proportional Hazards model and adjusted for relevant variables described above. We assessed the classification of risk using net integrated discrimination index. In addition, discriminative ability of various survival models was compared using the c‐statistic.29 Statistical analysis was performed using SPSS version 11.5 (SPSS Inc, Chicago, IL), Stata version 10.0 (StataCorp, College Station, TX), and R 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria). A P‐value of <0.05 was considered significant.
Results
The baseline data are shown in Tables 1 and 2. In the study, the vast majority (n=459, 86%) of the patients had AVA ≤1 cm2, while 72 (16%) had moderate AS (AVA [1–1.3 cm2]). There were no major clinical differences between these subgroups, except for higher age (72±12 versus 65±16 years), higher proportion of symptoms (87% versus 65%), higher STS score (11.5±5% versus 8.5±4%), and higher median BNP (145 versus 92 pg/mL) in those with severe versus moderate AS (all P<0.05). Similarly, indexed LV mass (119±45 versus 93±32 g/m2), mean aortic valve gradient (45±16 versus 22±10 mm Hg), and AVA (0.7±0.2 versus 1.2±0.1 cm2) were significantly worse in severe AS versus moderate AS (all P<0.01). Median LV‐GLS was −13.9% (interquartile range −16.3% to 11.5%), and slightly worse in severe versus moderate AS (−13.6% versus −14.2%, P=0.04). LV‐GLS had a statistically significant but weak association with LVEF (β −0.3, P<0.001) and LV‐SVI (β −0.20, P<0.001), indexed LV mass (β 0.19, P<0.001), and BNP (β 0.24, P<0.001). Similarly, there was a significant but weak association between BNP and LVEF (β −0.14, P=0.002) and indexed LV mass (β 0.20, P<0.001), but no association with LV‐SVI (β −0.04, P=0.3).
Table 1.
Baseline Characteristics of the Study Population
| Variable | Total Population (n=531) |
|---|---|
| Age, y | 71 (12) |
| Male sex | 58% |
| BSA, m2 | 0.3 |
| Angina | 34% |
| Syncope | 6% |
| NYHA Class | |
| I | 22% |
| II | 44% |
| III | 28% |
| IV | 7% |
| Any symptoms | 84% |
| Hypertension | 79% |
| Hyperlipidemia | 78% |
| Diabetes mellitus | 23% |
| Prior stroke | 8% |
| Smoking history | 51% |
| Obstructive CAD | 59% |
| Atrial fibrillation | 22% |
| Prior OHS | 23% |
| ICD | 4% |
| Pacemaker | 38% |
| Society of thoracic surgeons score | 11.(5) |
| β‐Blockers | 86% |
| ACE inhibitors | 45% |
| Aspirin | 90% |
| Statins | 74% |
| Diuretics | 89% |
| Aldosterone receptor blocker | 9% |
| Hemoglobin, mg/dL | 13 (2) |
| GFR, mL/min per 1.73 m2 | 73 (35) |
| LDL, mg/dL | 96 (40) |
| HDL, mg/dL | 50 (17) |
| Median BNP with IQL, pg/mL | 141 [60–313] |
| BNP quartiles | |
| 1st (0‐59) | 25% |
| 2nd (60–141) | 25% |
| 3rd (142–313) | 25% |
| 4th (>313) | 25% |
All continuous variables reported as mean (SD). ACE indicates angiotensin‐converting enzyme; BNP, brain natriuretic peptide; BSA, body surface area; CAD, coronary artery disease; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; ICD, internal cardioverter defibrillator; IQL, interquartile range; LDL, low‐density lipoprotein; NYHA, New York Heart Association; OHS, open heart surgery.
Table 2.
Echocardiographic Characteristics of the Study Population
| Variable | Total Population (n=531) |
|---|---|
| LV ejection fraction (%) | 58 (5) |
| Indexed LVEDD, cm/m2 | 2.3 (0.4) |
| Indexed LVESD, cm/m2 | 1.5 (0.4) |
| Indexed LA dimension, cm/m2 | 2.2 (0.5) |
| Indexed LV mass, g/m2 | 113 (38) |
| Diastolic dysfunction | |
| Abnormal relaxation | 87% |
| Pseudonormal | 12% |
| Restrictive filling | 1% |
| LVOT diameter, cm | 2.0±0.2 |
| AV gradient | |
| Peak, mm Hg | 74 (30) |
| Mean, mm Hg | 42 (18) |
| Calculated AV area (continuity equation) | 0.77 (0.2) |
| LV‐SVI, mL/m2 | 39 (10) |
| LV‐SVI <35 mL/m2 | 202 (38%) |
| Valvuloarterial impedance, mm Hg·mL·m−2 | 4.72 (1.4) |
| Aortic regurgitation | |
| None | 21% |
| Mild | 54% |
| Moderate | 25% |
| Mitral regurgitation | |
| None | 11% |
| Mild | 71% |
| Moderate | 18% |
| Tricuspid regurgitation | |
| None | 13% |
| Mild | 75% |
| Moderate | 12% |
| RVSP, mm Hg | 37 (13) |
| LV‐GLS (%) | −13.9 (3) |
| LV‐GLS quartiles | |
| 1st (> −16.3%) | 25% |
| 2nd (between (−16% to 3% and −14%) | 25% |
| 3rd (between −11.6% and −13.9%) | 24% |
| 4th (< −11.6%) | 26% |
All continuous variables reported as mean (SD). AV indicates aortic valve; EDD, end‐diastolic dimension; ESD, end‐systolic dimension; LA, left atrium; LV, left ventricle; LV‐GLS, left ventricular global longitudinal strain; LVOT, left ventricular outflow tract; LV‐SVI, left ventricular stroke volume index; RVSP, right ventricular systolic pressure.
Mean LV‐GLS values (%) for each BNP quartile were as follows: quartile 1 (−15.2±3), quartile 2 (−14.3±3), quartile 3 (−13.4±3), and quartile 4 (−12.6±4), P<0.001. The mean LV‐SVI values (mL/m2) for each LV‐GLS quartile were as follows: quartile 1 (41±10), quartile 2 (39±10), quartile 3 (39±10), and quartile 4 (35±9), P<0.001. The median BNP values (pg/mL) for LV‐GLS quartiles were as follows: quartile 1 (95 [41–212]), quartile 2 (109 [44–204]), quartile 3 (154 [60–307]), and quartile 4 (228 [97–429]), P<0.001. Finally, the mean LV‐SVI values for each BNP quartile were as follows: quartile 1 (40±11), quartile 2 (38±10), quartile 3 (38±10), and quartile 4 (38±9), P=0.4.
Overall, 405 patients (76%) underwent AVR and 126 (24%) were treated medically. Of the AVR patients, 179 (44%) underwent isolated surgical AVR, 18 (4%) underwent transcatheter AVR, and the rest underwent a combination procedure (AVR+ coronary artery bypass grafting +/− aortic surgery+/− mitral/tricuspid valve repair). There was no difference in LV‐GLS in patients requiring concomitant coronary artery bypass grafting (−13.6±3% versus −13.9±4%, respectively, P=0.1). The relevant parameters of the study sample, divided on whether they underwent AVR versus medical therapy, are shown in Table 3.
Table 3.
Relevant Characteristics of the Study Population, Separated on Basis of Aortic Valve replacement versus Medical therapy
| Variable | Medical Therapy (n=126) | AVR (n=405) | P Value |
|---|---|---|---|
| Age, y | 73±13 | 71±12 | 0.05 |
| Male gender | 51% | 60% | 0.04 |
| Angina | 26% | 38% | 0.01 |
| Syncope | 7% | 6% | 0.1 |
| NYHA Class | |||
| I | 45% | 18% | <0.001 |
| II | 33% | 46% | |
| III | 15% | 29% | |
| IV | 2% | 7% | |
| Hypertension | 78% | 79% | 0.5 |
| Prior stroke | 9% | 8% | 0.6 |
| Obstructive CAD | 47% | 63% | 0.001 |
| Atrial fibrillation | 19% | 23% | 0.2 |
| Prior OHS | 23% | 23% | 0.5 |
| Society of thoracic surgeons score | 11.6±5 | 10.9±6 | 0.3 |
| β‐Blockers | 74% | 90% | <0.001 |
| ACE inhibitors | 49% | 43% | 0.1 |
| Aspirin | 72% | 95% | <0.001 |
| Statins | 65% | 77% | <0.001 |
| GFR, mL/min per 1.73 m2 | 69±35 | 74±30 | 0.1 |
| Median BNP with IQL, pg/mL | 126 (56–264) | 171 (81–546) | <0.001 |
| LV ejection fraction (%) | 57±5 | 57±5 | 0.6 |
| Indexed LV mass, g/m2 | 112±37 | 114±38 | 0.1 |
| AV gradient | |||
| Peak, mm Hg | 55±29 | 80±27 | <0.001 |
| Mean, mm Hg | 31±18 | 46±16 | <0.001 |
| Calculated AV area (continuity equation) | 0.92±0.2 | 0.72±0.2 | <0.001 |
| LV‐stroke volume index, mL/m2 | 38±9 | 39±10 | 0.4 |
| Valvuloarterial impedance, mm Hg·mL−1·m2 | 4.6±1.2 | 4.7±1.5 | 0.1 |
| RVSP, mm Hg | 36±11 | 36±11 | 0.6 |
| LV‐GLS (%) | −13.8±4 | −13.9±4 | 0.9 |
ACE indicates angiotensin‐converting enzyme; AVR, aortic valve replacement; BNP, brain natriuretic peptide; CAD, coronary artery disease; GFR, glomerular filtration rate; IQL, interquartile range; LV‐GLS, left ventricular global longitudinal strain; NYHA, New York Heart Association; OHS, open heart surgery; RVSP, right ventricular systolic pressure.
Outcomes and Survival Data
During 4.7±2 years of follow‐up, mortality was observed in 161 (30%) patients (6 [1%] deaths within 30 days post‐AVR). The breakdown of deaths was as follows: 94 (58%) cardiac, 17 (11%) documented noncardiac, and 49 (31%) unknown (however, none of them had a clearly documented noncardiac etiology to account for death). The proportion of deaths was similar between severe and moderate AS (137 [30%] versus 24 [33%]), with no difference in survival during follow‐up (P=0.1).
Results of multivariable Cox Proportional Hazard Survival analysis (for all‐cause mortality) are shown in Table 4A and 4B. The χ2 for all‐cause mortality incrementally increased as follows: STS score 31, STS+BNP 77, STS+BNP+LV‐GLS 120, and STS+BNP+LV‐GLS+AVR 140, all P<0.001. Using the integrated discrimination index, we further demonstrate that addition of BNP and LV‐GLS improved risk stratification for mortality. The results are shown in Figure 1. The ability of various models to predict mortality incrementally increased as follows: c‐statistic for STS score was 0.60 (0.58–0.64), for STS score+BNP was 0.67 (0.62–0.70), and STS score+ BNP+LV‐GLS was 0.74 (0.68–0.78). The c‐statistic for STS score+BNP+LV‐GLS+AVR further increased to 0.79 (0.72–0.84), all P<0.01.
Table 4.
Multivariable Cox Proportional Hazard Analysis for All‐Cause Mortality in the Study Population
| Variable | Hazard Ratio | P Value |
|---|---|---|
| (A) Variables listed below entered in a stepwise fashiona | ||
| Age (10‐year increase) | 1.46 (1.12–1.92) | 0.003 |
| NYHA Class | 1.27 (1.05–1.54) | 0.03 |
| Coronary artery disease | 1.72 (1.20–2.46) | <0.001 |
| Glomerular filtration rate (for every 10‐unit decrease) | 1.15 (1.08–1.22) | <0.001 |
| BNP (for every 10 pg/mL increase) | 1.16 (1.09–1.23) | <0.001 |
| Left ventricular global longitudinal strain (for every unit worsening) | 1.13 (1.07–1.18) | <0.001 |
| Aortic valve surgery (time‐dependent covariate analysis) | 0.34 (0.23–0.48) | <0.001 |
| (B) STS score entered in the modelb | ||
| Society of Thoracic Surgeons (STS) score | 1.05 (1.03–1.07) | <0.001 |
| BNP (for every 10 pg/mL increase) | 1.14 (1.08–1.22) | <0.001 |
| Left ventricular global longitudinal strain (for every unit worsening) | 1.09 (1.04–1.15) | <0.001 |
| Aortic valve surgery (time‐dependent covariate analysis) | 0.34 (0.24–0.48) | <0.001 |
In Part (A), the following variables were considered for analysis: age, sex, symptoms, comorbidities, pacemaker, defibrillator, medications, indexed left ventricular mass and systolic dimension, left atrial volume index, ejection fraction, diastolic function, stroke volume index, aortic valve area, aortic valve mean gradient, aortic and mitral regurgitation, global longitudinal strain, brain natriuretic peptide (BNP), aortic valve surgery, and type and time of surgery. NYHA indicates New York Heart Association.
In Part (B), variables that constitute STS score were not considered for analysis. Other variables are similar to Part (A). Because of collinearity, only stroke volume index (and not valvuloarterial impedance) was considered for the model. Results are similar if valvuloarterial impedance was considered.
Figure 1.
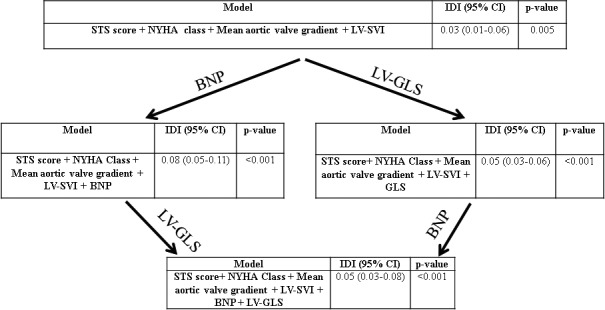
Reclassification of mortality risk in the study sample, based on various models. BNP indicates brain natriuretic peptide; GLS, global longitudinal strain; IDI, integrated discrimination index; LV‐SVI, left ventricular stroke volume index; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons.
The proportion of all‐cause deaths, separated on the basis of BNP quartiles was as follows: quartile 1 (14 [11%]), quartile 2 (34 [25%]), quartile 3 (42 [32%]), and quartile 4 (71 [54%]). Figure 2A illustrates the adjusted survival curves stratified according to increasing BNP quartiles (P<0.001). The proportion of deaths, separated on the basis of LV‐GLS quartiles was as follows: quartile 1 (22 [17%]), quartile 2 (33 [25%]), quartile 3 (39 [31%]), and quartile 4 (67 [47%]). Figure 2B illustrates the adjusted survival curves stratified according to worsening LV‐GLS quartiles (P<0.001).
Figure 2.
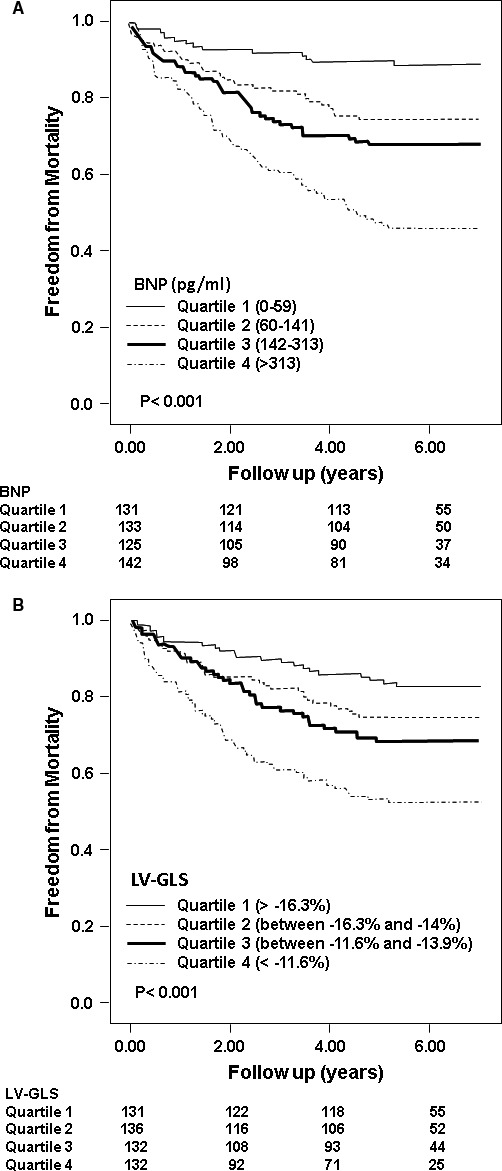
Adjusted survival curves demonstrating outcomes based on various quartiles of (A) brain natriuretic peptide (BNP) and (B) left ventricular global longitudinal strain (LV‐GLS).
Subsequently, in order to understand the interplay between LV‐GLS and BNP, we created 4 subgroups, based on medians. The proportion of deaths, based on these 4 subgroups, were as follows: (1) LV‐GLS≥median (ie, better value) and BNP <median (21/161 [13%]); (2) LV‐GLS ≥median, BNP≥median (33/102 [32%]); (3) LV‐GLS <median, BNP < median (27/106 [26%]); and (4) LV‐GLS <median (ie, worse value) and BNP ≥median (80/162 [49%]). Figure 3 illustrates the survival curves according to LV‐GLS and BNP medians (P<0.001).
Figure 3.
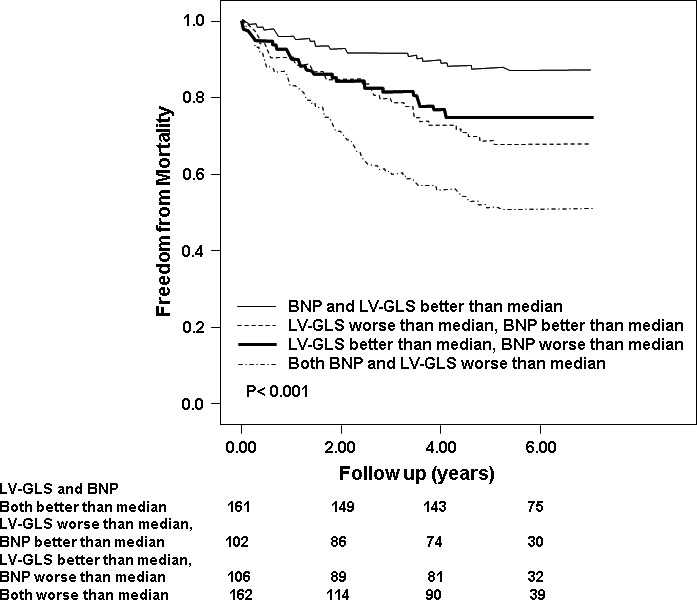
Adjusted survival curves demonstrating outcomes based on 4 subgroups derived based on brain natriuretic peptide (BNP) and left ventricular global longitudinal strain (LV‐GLS) levels better or worse than median.
The breakdown of deaths, according to symptoms and LV‐GLS/BNP was as follows: asymptomatic and both, LV‐GLS and BNP better than median (5/51 or 10%), asymptomatic and 1 or both, LV‐GLS and BNP worse than median (20/64 or 31%), symptomatic and both, LV‐GLS and BNP better than median (16/110 or 15%) and symptomatic, and 1 or both, LV‐GLS and BNP worse than median (120/306 or 39%). Figure 4 illustrates the survival curves according to symptom status and whether BNP/LV‐GLS were better or worse than median (P<0.001). Even in asymptomatic patients, the mortality was significantly high in the setting of 1 or both, LV‐GLS and BNP worse than median.
Figure 4.
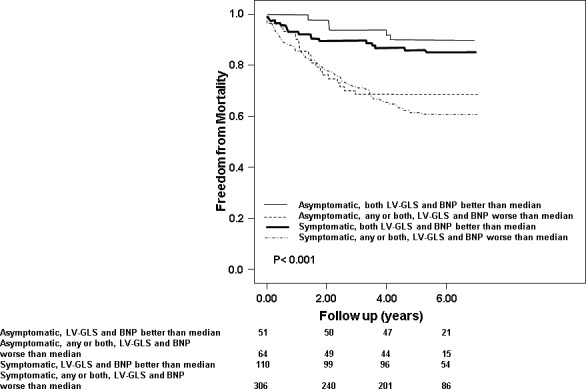
Adjusted survival curves demonstrating outcomes of 4 subgroups, based on whether both brain natriuretic peptide (BNP) and left ventricular global longitudinal strain (LV‐GLS) were better than median or 1/both were worse than median and symptoms.
The breakdown of all‐cause deaths, according to median STS score (median 7.3 [3.9–12.9]) and LV‐GLS/BNP was as follows: STS score <median and both, LV‐GLS and BNP better than median (8/79 or 10%), STS score <median and 1 or both, LV‐GLS and BNP worse than median (52/184 or 28%), STS score ≥median and both, LV‐GLS and BNP better than median (13/81 or 16%) and STS score ≥median, and 1 or both, LV‐GLS and BNP worse than median (88/187 or 47%). Figure 5 illustrates the survival curves of patients stratified according to STS score and whether BNP/LV‐GLS were better or worse than median (P<0.001). Even in patients with STS scores lower than median, the mortality was significantly high in the setting of 1 or both, LV‐GLS and BNP worse than median.
Figure 5.
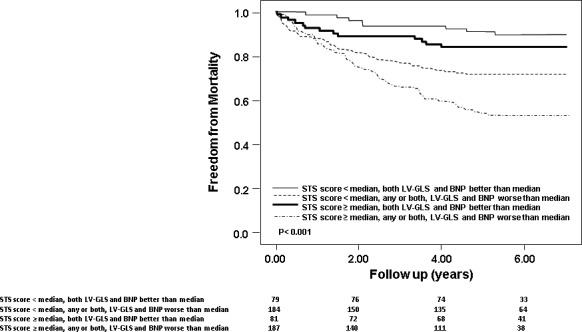
Adjusted survival curves demonstrating outcomes of 4 subgroups, based on whether both brain natriuretic peptide (BNP) and left ventricular global longitudinal strain (LV‐GLS) were better than median or 1/both were worse than median, and STS score better or worse than median.
Multivariable Cox Proportional Hazard Survival analysis, for the secondary outcome (cardiac mortality and death due to unknown causes, excluding noncardiac deaths, n=143) demonstrated that increasing STS score (hazard ratio 1.05 [1.01–1.09]), every 10 pg/mL increase in BNP (1.08 [1.06–1.11]), every unit worsening of LV‐GLS (hazard ratio 1.12 [1.06–1.18]), and AV surgery (0.36 [0.24–0.52]) were independent predictors (χ2 for the model 104, P<0001).
Discussion
In our observational study of contemporary patients with significant AS and preserved LVEF, we demonstrate that increasing BNP levels and worsening LV‐GLS were independent predictors of mortality, providing additive (rather than duplicative) prognostic utility. Furthermore, using integrated discrimination improvement, we demonstrate that addition of BNP and LV‐GLS further improved our ability to reclassify mortality risk in AS patients. It appears that LV‐GLS and BNP could potentially help us identify patients who could benefit from earlier AVR. We included patients with patients with AVA 1.0 to 1.3 cm2 because AS is a continuum and we wanted to evaluate survival of these patients vis‐a‐vis current therapeutic techniques. Asymptomatic patients had significantly worse survival, in the setting of abnormal LV‐GLS and/or BNP. This impact on survival was also seen in the subgroup with low STS scores, where patients with LV‐GLS and/or BNP worse than median had significantly worse outcomes versus those with normal LV‐GLS and BNP. However, the study is potentially underpowered to make conclusive assertions about subgroup analyses; and a larger, prospective study is needed to be conclusively assertive.
In the current study, when BNP and LV‐GLS were considered for survival analysis, known predictor such as LV‐SVI did not maintain statistical significance. This is likely because sensitive markers such as LV‐GLS and BNP become abnormal earlier in the disease cascade, as compared to flow‐dependent markers such as LV‐SVI. Additionally, there are known inherent technical limitations in measuring LV‐SVI, which takes LVOT area into account. As previously described, LVOT area can be potentially inaccurate on 2‐dimensional echocardiography when compared to gated computed tomographic techniques.30 This potentially generates erroneous LV‐SVI values, which results in misclassifying AS patients into different strata with varying risk profiles.
In patients with AS, in order to compensate for increased wall stress and preserve LVEF, there is progression of LV hypertrophy. However, LVEF eventually drops and in this setting, if AVR is not performed, there is a significant reduction in survival. Therefore, objective and sensitive parameters that identify early LV dysfunction, prior to a drop in LVEF could potentially have a big impact on appropriate timing of surgery and in turn, potential survival. BNP is released in response to increased ventricular wall stress, and our data are in agreement with previous studies that have shown that BNP levels correlate with survival.8, 10, 11, 12, 13, 14 However, BNP is nonspecific, with multiple clinical situations resulting in elevated values. Also, as demonstrated in the current study, BNP levels appear to be lower in AS patients in particular (and valvular heart disease in general) than in other etiologies of heart failure. Hence, different BNP thresholds may be needed in valvular heart disease to adequately predict outcomes. A previous report has suggested the use of different thresholds, based on age and sex.31 A recent report utilized BNP ratios generated based on these thresholds and demonstrated incremental prognostic utility of BNP in the setting of significant AS.16 LV‐GLS is much more sensitive in detecting subtle abnormalities in myocardial mechanics and may indicate pathology before evident on conventional indices of LV function. Previous studies have indeed demonstrated that impairment in LV‐GLS can occur even in the setting of a preserved LVEF, due to subendocardial ischemia and fibrosis.32, 33 Additionally, reduced LV‐GLS is associated with poorer outcomes in patients with significant AS,28, 34 with preoperative LV‐GLS an independent predictor of postoperative outcomes.11 Using these markers provides synergistic risk stratification in patients with significant AS prior to onset of overt LV systolic dysfunction or symptoms.
Clinical Implications
In patients with significant AS and preserved LVEF, a combination of BNP and LV‐GLS provides synergistic risk stratification, independent of symptoms, risk factors, and echocardiographic variables. Prospective studies are needed to determine whether onset of changes in these parameters rather than waiting for symptoms or onset of abnormal LVEF may be more appropriate to determine valve intervention timing. Additionally, a risk score, incorporating these markers alongside other clinical and echocardiographic markers, could be developed and prospectively validated in asymptomatic patients with significant AS.
Limitations
This was an observational retrospective study conducted at a large tertiary care center and is likely not free from referral bias. Not all patients with severe AS seen at our institution had BNP levels obtained in close proximity to the echocardiogram. However, the baseline characteristics of the current study population were similar to those that did not have BNP levels measured. During follow‐up, only a small proportion of patients underwent isolated AVR, making this a heterogeneous population, where other factors such as coronary artery disease and aortic disease could have affected outcomes. However, AS patients tend to be typically older with many comorbidities, and our study reflects the current state of practice in most valve centers. The biggest utility of these newer markers would potentially be in asymptomatic patients with significant AS to determine appropriate timing of surgery, and not in those with symptoms who already would meet criteria for surgery. However, the study is potentially underpowered to make conclusive assertions about this specific subgroup. We report all‐cause mortality as the primary end point, as opposed to cardiac mortality. However, on secondary outcomes analysis, where documented noncardiac deaths were excluded, the basic results were similar.
Conclusions
In patients with significant AS and preserved LVEF, a combination of BNP and LV‐GLS predicts mortality. Assessment of LV‐GLS and BNP could have a potential role in synergistic improvement in risk stratification of AS patients with a preserved LVEF, especially those perceived to be without symptoms and/or deemed at a low risk. Future prospective studies are needed to confirm these observations.
Disclosures
None relevant to the manuscript. Dr Sabik is a consultant for Medtronic and Sorin.
(J Am Heart Assoc. 2016;5:e002561 doi: 10.1161/JAHA.115.002561)
References
- 1. Ross J Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38:61–67. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 3. Ozkan A, Hachamovitch R, Kapadia SR, Tuzcu EM, Marwick TH. Impact of aortic valve replacement on outcome of symptomatic patients with severe aortic stenosis with low gradient and preserved left ventricular ejection fraction. Circulation. 2013;128:622–631. [DOI] [PubMed] [Google Scholar]
- 4. Pibarot P, Dumesnil JG. Low‐flow, low‐gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845–1853. [DOI] [PubMed] [Google Scholar]
- 5. Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Senechal M, Pibarot P. Outcome of patients with aortic stenosis, small valve area, and low‐flow, low‐gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1259–1267. [DOI] [PubMed] [Google Scholar]
- 6. Parikh R, Goodman AL, Barr T, Sabik JF, Svensson LG, Rodriguez LL, Lytle BW, Grimm RA, Griffin BP, Desai MY. Outcomes of surgical aortic valve replacement for severe aortic stenosis: incorporation of left ventricular systolic function and stroke volume index. J Thorac Cardiovasc Surg. 2015;149:1558–1566.e1551. [DOI] [PubMed] [Google Scholar]
- 7. Pierard S, de Meester C, Seldrum S, Pasquet A, Gerber B, Vancraeynest D, Robert A, El Khoury G, Noirhomme P, Vanoverschelde JL. Impact of preoperative symptoms on postoperative survival in severe aortic stenosis: implications for the timing of surgery. Ann Thorac Surg. 2014;97:803–809. [DOI] [PubMed] [Google Scholar]
- 8. Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. [DOI] [PubMed] [Google Scholar]
- 9. Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J. 2005;26:1309–1313. [DOI] [PubMed] [Google Scholar]
- 10. Monin JL, Lancellotti P, Monchi M, Lim P, Weiss E, Pierard L, Gueret P. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation. 2009;120:69–75. [DOI] [PubMed] [Google Scholar]
- 11. Dahl JS, Videbaek L, Poulsen MK, Rudbaek TR, Pellikka PA, Moller JE. Global strain in severe aortic valve stenosis: relation to clinical outcome after aortic valve replacement. Circ Cardiovasc Imaging. 2012;5:613–620. [DOI] [PubMed] [Google Scholar]
- 12. Bergler‐Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, Binder T, Pacher R, Maurer G, Baumgartner H. Natriuretic peptides predict symptom‐free survival and postoperative outcome in severe aortic stenosis. Circulation. 2004;109:2302–2308. [DOI] [PubMed] [Google Scholar]
- 13. Ben‐Dor I, Minha S, Barbash IM, Aly O, Dvir D, Deksissa T, Okubagzi P, Torguson R, Lindsay J, Satler LF, Pichard AD, Waksman R. Correlation of brain natriuretic peptide levels in patients with severe aortic stenosis undergoing operative valve replacement or percutaneous transcatheter intervention with clinical, echocardiographic, and hemodynamic factors and prognosis. Am J Cardiol. 2013;112:574–579. [DOI] [PubMed] [Google Scholar]
- 14. Nessmith MG, Fukuta H, Brucks S, Little WC. Usefulness of an elevated B‐type natriuretic peptide in predicting survival in patients with aortic stenosis treated without surgery. Am J Cardiol. 2005;96:1445–1448. [DOI] [PubMed] [Google Scholar]
- 15. Bergler‐Klein J, Mundigler G, Pibarot P, Burwash IG, Dumesnil JG, Blais C, Fuchs C, Mohty D, Beanlands RS, Hachicha Z, Walter‐Publig N, Rader F, Baumgartner H. B‐type natriuretic peptide in low‐flow, low‐gradient aortic stenosis: relationship to hemodynamics and clinical outcome: results from the multicenter truly or pseudo‐severe aortic stenosis (TOPAS) study. Circulation. 2007;115:2848–2855. [DOI] [PubMed] [Google Scholar]
- 16. Clavel MA, Malouf J, Michelena HI, Suri RM, Jaffe AS, Mahoney DW, Enriquez‐Sarano M. B‐type natriuretic peptide clinical activation in aortic stenosis: impact on long‐term survival. J Am Coll Cardiol. 2014;63:2016–2025. [DOI] [PubMed] [Google Scholar]
- 17. Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112:I377–I382. [DOI] [PubMed] [Google Scholar]
- 18. Lancellotti P, Moonen M, Magne J, O'Connor K, Cosyns B, Attena E, Donal E, Pierard L. Prognostic effect of long‐axis left ventricular dysfunction and B‐type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol. 2010;105:383–388. [DOI] [PubMed] [Google Scholar]
- 19. Gerber IL, Stewart RA, Legget ME, West TM, French RL, Sutton TM, Yandle TG, French JK, Richards AM, White HD. Increased plasma natriuretic peptide levels reflect symptom onset in aortic stenosis. Circulation. 2003;107:1884–1890. [DOI] [PubMed] [Google Scholar]
- 20. Weber M, Arnold R, Rau M, Brandt R, Berkovitsch A, Mitrovic V, Hamm C. Relation of N‐terminal pro‐B‐type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol. 2004;94:740–745. [DOI] [PubMed] [Google Scholar]
- 21. Lim P, Monin JL, Monchi M, Garot J, Pasquet A, Hittinger L, Vanoverschelde JL, Carayon A, Gueret P. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B‐type natriuretic peptide. Eur Heart J. 2004;25:2048–2053. [DOI] [PubMed] [Google Scholar]
- 22. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 23. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23; quiz 101–102. [DOI] [PubMed] [Google Scholar]
- 24. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 25. Minners J, Allgeier M, Gohlke‐Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008;29:1043–1048. [DOI] [PubMed] [Google Scholar]
- 26. Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–298. [DOI] [PubMed] [Google Scholar]
- 28. Kusunose K, Goodman A, Parikh R, Barr T, Agarwal S, Popovic ZB, Grimm RA, Griffin BP, Desai MY. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging. 2014;7:938–945. [DOI] [PubMed] [Google Scholar]
- 29. Uno H, Cai T, Pencina MJ, D'Agostino RB, Wei LJ. On the C‐statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Brien B, Schoenhagen P, Kapadia SR, Svensson LG, Rodriguez L, Griffin BP, Tuzcu EM, Desai MY. Integration of 3D imaging data in the assessment of aortic stenosis: impact on classification of disease severity. Circ Cardiovasc Imaging. 2011;4:566–573. [DOI] [PubMed] [Google Scholar]
- 31. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. [DOI] [PubMed] [Google Scholar]
- 32. Delgado V, Tops LF, van Bommel RJ, van der Kley F, Marsan NA, Klautz RJ, Versteegh MI, Holman ER, Schalij MJ, Bax JJ. Strain analysis in patients with severe aortic stenosis and preserved left ventricular ejection fraction undergoing surgical valve replacement. Eur Heart J. 2009;30:3037–3047. [DOI] [PubMed] [Google Scholar]
- 33. Becker M, Kramann R, Dohmen G, Luckhoff A, Autschbach R, Kelm M, Hoffmann R. Impact of left ventricular loading conditions on myocardial deformation parameters: analysis of early and late changes of myocardial deformation parameters after aortic valve replacement. J Am Soc Echocardiogr. 2007;20:681–689. [DOI] [PubMed] [Google Scholar]
- 34. Yingchoncharoen T, Gibby C, Rodriguez LL, Grimm RA, Marwick TH. Association of myocardial deformation with outcome in asymptomatic aortic stenosis with normal ejection fraction. Circ Cardiovasc Imaging. 2012;5:719–725. [DOI] [PubMed] [Google Scholar]


