Abstract
Background
Cigarette smoking is a risk factor for stroke, but the mechanisms by which smoking contributes to stroke are not well understood. This study aimed to evaluate the roles of lung function (represented by forced expiratory volume in the first second (FEV1)) and aldosterone as potential mediators of the association of smoking with stroke.
Methods and Results
The data were derived from 5010 Jackson Heart Study participants who had mean follow‐up of 97.9 months. Using the Cox proportional hazards model, we estimated the hazard ratios of smoking for total stroke with and without adjustment for FEV1 and/or aldosterone at baseline after controlling for the confounders. The hazard ratio for current smoking (versus never smoking) was 2.70 (95% CI 1.71 to 4.25) for total stroke after adjustment for the confounders. Additional adjustment for FEV1 and aldosterone reduced the hazard ratio to 2.32 (95% CI 1.42 to 3.79), suggesting that 22.4% of the excess risk of current smoking for total stroke is mediated by these factors. FEV1 and aldosterone account for 13.1% and 12.1%, respectively, of the excess risk. The hazard ratio for FEV1 increased (0.61 versus 0.65) after including systemic inflammatory marker C‐reactive protein, and the hazard ratios for aldosterone were comparable for the models that included all confounders and smoking status with or without different blood pressure measurements.
Conclusions
Our findings suggest that the difference in stroke risk between current and never smokers may develop partially through pathways involving lung function and aldosterone and that the mediation effect through aldosterone is independent of blood pressure.
Keywords: aldosterone, lung function, mediation effects, smoking, stroke
Introduction
Stroke occurs when the blood supply to the brain is blocked or a blood vessel in the brain ruptures, causing brain tissue to die.1, 2 There are many risk factors for stroke, and some cannot be changed, such as age, sex, race, ethnicity, and heredity, whereas others are modifiable, including high blood pressure, elevated cholesterol, sickle cell disease, obesity, and smoking.3, 4 Numerous global studies established cigarette smoking as a risk factor for stroke across various ethnicities and populations.5, 6, 7, 8, 9, 10
Multiple possible mechanisms underlie the association between cigarette smoking and risk of stroke. Toxic chemicals inhaled from smoking may contribute to stroke by affecting lung function. It is well known that continuing smokers have an average rate of decline in forced expiratory volume in the first second (FEV1) that is substantially greater than that of people who have never smoked,11, 12, 13, 14 and this impaired pulmonary function is associated with an increased risk of cardiovascular diseases,15, 16, 17 possibly through an inflammatory mechanism.18, 19
Smoking may also increase the risk of stroke by causing direct damage to the vasculature, altering both its architecture and function20, 21 or having effects on hemodynamic factors within the circulation.22, 23 The hemodynamic effects of cigarette smoking in normotensive participants include increases in blood pressure and pulse rate.20 Plasma aldosterone levels increase significantly after smoking,24 and enhanced activation of the renin–angiotensin–aldosterone system is observed in chronic cigarette smokers.25 By regulating sodium and potassium levels, aldosterone helps control vascular tone and intravascular volume; consequently, elevated levels of aldosterone can result in increases in blood pressure, leading to hypertension, which is a major risk factor for stroke.26, 27 Elevated aldosterone may also increase the risk of stroke independent of its effects on blood pressure.28
Evidence from some observational studies showed that FEV1 decline and elevated aldosterone are associated with cigarette smoking11, 12, 13, 14, 24 and higher risk of stroke.15, 16, 17, 27, 28 These results suggest that differences in aldosterone and FEV1 between smokers and nonsmokers may exist, but the extent to which this relationship explains the stroke risk difference is unclear. In this study, we assessed whether decreased FEV1 and higher serum aldosterone levels were associated with a higher risk of stroke among current smokers versus never smokers and then applied related mediation analysis techniques to quantify the mediation effect magnitude through FEV1 and aldosterone and illustrated the possible mechanisms by which these mediation effects were realized.
Methods
Study Population
Data for this study were collected as part of the Jackson Heart Study (JHS), the largest single‐site, prospective, epidemiological investigation of cardiovascular disease among African Americans. The study enrolled 5301 participants recruited from urban and rural areas of the 3 counties (Hinds, Madison, and Rankin) that make up the Jackson, Mississippi, metropolitan statistical area. The participants underwent a baseline examination between September 2000 and March 2004 to collect data regarding demographic information, socioeconomic characteristics, medical history, physical examination, laboratory measurements, cardiac results, and medications. Ongoing cohort surveillance includes abstraction of medical records and death certificates for relevant International Classification of Diseases codes and adjudication of nonfatal events and deaths through 2011. Details of the study design and recruitment protocol have been described elsewhere.29, 30 All JHS participants gave written informed consent, and the study was approved by institutional review boards of the participating institutions: Jackson State University, Tougaloo College, and the University of Mississippi Medical Center.
Main Exposure
Current smokers were defined as participants who gave a positive response to the questions, “Have you smoked more than 400 cigarettes in your lifetime?” and “Do you now smoke cigarettes?” Past smoking was defined as a positive response to the first question and a negative response to the second question. Never smokers were those who responded no to the first question.
Confounders
We used baseline self‐reported data on physical activity (ideal status defined as ≥150 min/week at moderate intensity or ≥75 min/week at vigorous intensity or a combination based on American Heart Association physical activity classification)31; alcohol consumption in the past 12 months; family history of high blood pressure, stroke, or heart disease (from either father or mother); and the number of years of schooling completed (dichotomized as less than or more than high school).
Potential Mediators
Pulmonary function, including FEV1, was measured at baseline using computerized spirometry; maximum values of FEV1 were selected for analysis based on recommendations from the American Thoracic Society.32 Baseline serum aldosterone was measured by radioimmunoassay (Siemens), and serum C‐reactive protein (the marker of systemic inflammation used in this study) was measured by the latex particle immunoturbidimetric assay (Roche Diagnostics).33 Diastolic and systolic blood pressures were measured using a Hawksley random‐zero sphygmomanometer (Hawsley and Sons Ltd), and mean arterial pressure was calculated using the formula (SBP/3+2×DBP/3), in which DBP is diastolic blood pressure and SBP is systolic blood pressure.34
Outcome
The primary outcome of interest in this study was incident total stroke (ischemic and hemorrhagic combined). Trained interviewers conduct annual follow‐up telephone interviews to ascertain any significant health events since the last JHS contact, including diagnostic tests, hospitalizations, or death. Information on cohort hospitalizations and deaths is transmitted to the medical record abstraction unit, which reviews death certificates and hospital records to identify cardiovascular disease events including stroke in the cohort. A computer‐generated diagnosis with follow‐up review and physician adjudication completes final hospitalized stroke event classification.35
Statistical Analyses
Continuous variables are presented as mean (standard deviation), and categorical variables are expressed as proportions. Baseline characteristics and potential mediators were compared with chi‐square tests or 1‐way ANOVA with respect to smoking status. Multiple linear regression was used to model smoking status–mediator relations in JHS participants, with adjustment for confounders. Multivariate survival analysis using the Cox proportional hazards regression model was performed for categorical and continuous predictor variables (exposure, mediators, and confounders) to yield hazard ratios (HRs) and 95% CIs.
We conceptualized the hypothesized pathways and decomposed the total effect of cigarette smoking on the risk of incident stroke into the direct and indirect effects mediated by FEV1 and aldosterone (Figure 1). To assess the serum aldosterone concentration (ng/dL) and FEV1 at baseline of the JHS as potential mediators of the smoking–incident stroke relationship, we estimated the total effect of smoking status on incident stroke with adjustment for the confounders including age, sex, physical activity, alcohol consumption, family history of related diseases, and education. We then added the 2 postulated mediators to the model separately or combined. A change in HRs for smoking status was taken as evidence of mediation through the considered mediators.36, 37 Other risk factors for stroke such as obesity, hypertension, diabetes, and cholesterol were assessed and entered the test models at a later stage as additional potential mediators. Similar methods were extended to assess whether FEV1 mediated the smoking–stroke relationship by an inflammatory mechanism and whether the mediation effects through elevated serum aldosterone were blood pressure dependent. The decomposition of mediation effects through FEV1 or aldosterone was further confirmed using the linear structural equation modeling approach38, 39 (see Data S1, Figure S1 and S2).
Figure 1.
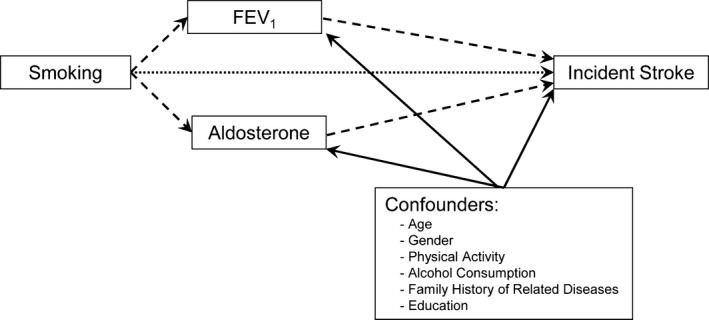
Decomposition of the total effect of cigarette smoking on the risk of incident stroke into the direct and indirect effects as mediated by FEV1 and aldosterone. The dotted arrow represents the direct effect, the dashed arrow represents the indirect effect, and the solid arrows represent sources of noncausal association of multiple confounders with the potential mediators and the outcome. FEV1 indicates forced expiratory volume in the first second.
The percentage at which the mediators explain the smoking status difference on incident stroke was computed using the following equation37, 40
All statistical tests were 2‐tailed, and P<0.05 was considered significant. The SAS software package (version 9.4; SAS Institute) was used for all analyses.
Results
Of 5301 participants enrolled in the JHS, we excluded participants with prior stroke (n=241) and those missing smoking status (n=50). The final analysis included 5010 participants who were followed from JHS visit 1 (2000–2004) to December 31, 2011. During a mean of 97.9 months of follow‐up (range 0.6 to 134.2 months), a total of 156 stroke events occurred, of which 143 were ischemic and 13 were hemorrhagic. Baseline characteristics of study participants are presented in Table 1. Compared with patients who had never smoked, past and current smokers were more likely to be men, to consume alcohol, and to be less educated. Blood pressure was slightly higher in current smokers than in never smokers, and current smokers tended to report less physical activity and to have shorter follow‐up time than never smokers and past smokers.
Table 1.
Baseline Characteristics of Jackson Heart Study Participants by Smoking Status
| Variable | Never Smokers (n=3448) | Past Smokers (n=910) | Current Smokers (n=652) | P Valuea |
|---|---|---|---|---|
| Age, y | 54.1±13.2 | 60.1±11.1 | 52.3±11.1 | <0.001 |
| Sex, male, % | 30.5 | 48.4 | 50.2 | <0.001 |
| Ideal health indicator via physical activityb, % | 19.8 | 21.3 | 15.3 | 0.010 |
| Alcohol consumption in the past 12 months, % | 41.4 | 49.0 | 71.5 | <0.001 |
| Family history of high blood pressure, stroke, or heart disease, % | ||||
| Father | 49.9 | 47.6 | 48.5 | 0.496 |
| Mother | 68.8 | 68.3 | 69.1 | 0.946 |
| Either father or mother | 79.1 | 78.8 | 79.1 | 0.972 |
| Education, less than high school, % | 16.1 | 27.5 | 24.8 | <0.001 |
| Blood pressure, mm Hg | ||||
| Systolic | 126.2±18.2 | 127.7±18.0 | 128.5±19.3 | 0.003 |
| Diastolic | 79.0±10.4 | 77.7±10.3 | 80.0±11.0 | <0.001 |
| MAP | 94.7±11.3 | 94.4±11.0 | 96.1±12.2 | 0.009 |
| Hypertension, % | 57.1 | 68.5 | 56.5 | <0.001 |
| hs‐CRP, mg/L | 4.9±7.4 | 4.9±7.8 | 6.3±1.6 | 0.103 |
| Follow‐up, months | 98.5±20.9 | 98.7±23.5 | 93.5±26.7 | <0.001 |
Continuous values are presented as mean±SD, and all other values are frequencies. hs‐CRP indicates high‐sensitivity C‐reactive protein; MAP, mean arterial pressure.
Chi‐square test or ANOVA was used to compare baseline characteristics and potential mediators of participants by smoking status.
≥150 min/week at moderate intensity or ≥75 min/week at vigorous intensity or a combination based on American Heart Association physical activity classification.
Multivariate linear regression was conducted for the relationship between smoking status and FEV1 or serum aldosterone concentration (ng/dL) (Table 2). With never smoking as the reference group, current smoking was negatively associated with FEV1 and positively associated with serum aldosterone after adjusting for other baseline confounders (age; sex; physical activity; alcohol consumption in the past 12 months; family history of high blood pressure, stroke, or heart disease; and education). No statistically significant associations were found between past smoking and FEV1 or serum aldosterone.
Table 2.
Linear Regression Coefficient of Smoking Status Predicting FEV1 and Serum Aldosterone Among Jackson Heart Study Participants
| Outcome Variable | Past Smokers (n=910) | Current Smokers (n=652) | ||||
|---|---|---|---|---|---|---|
| β1 a | 95% CI | P Value | β2 a | 95% CI | P Value | |
| FEV1, L | 0.019 | −0.019 to 0.057 | 0.317 | −0.143 | −0.187 to −0.099 | <0.001 |
| Serum aldosterone, ng/dL | 0.250 | −0.181 to 0.681 | 0.255 | 0.494 | −0.001 to 0.990 | 0.050 |
FEV1 indicates forced expiratory volume in the first second.
Multivariate regression was adjusted by confounders (age; sex; physical activity; alcohol consumption in the past 12 months; family history of high blood pressure, stroke, or heart disease; and education).
Table 3 shows the results for Cox regression analysis of 2 potential mediators for incident stroke. In multivariate analysis, FEV1 was associated with a lower risk of total stroke (HR 0.61 per 1‐L increase of FEV1, 95% CI 0.42 to 0.89) after adjustment for smoking status and other baseline confounders. In contrast, the risk of total incident stroke increased by 15% per 5‐ng/dL increase in serum aldosterone (HR 1.15, 95% CI 1.10 to 1.21) after adjustment for smoking status and other baseline confounders.
Table 3.
Cox Regression Analysis Between Potential Mediators (FEV1 and Aldosterone) and Incident Stroke Among Jackson Heart Study Participants
| Variable | Hazard Ratioa | 95% CI | P Value |
|---|---|---|---|
| FEV1, L | 0.61 | 0.42 to 0.89 | 0.010 |
| Serum aldosterone, ng/dLb | 1.15 | 1.10 to 1.21 | <0.001 |
FEV1 indicates forced expiratory volume in the first second.
Hazard ratio for FEV1 and aldosterone from the Cox models after adjusting for smoking status and other confounders (age; sex; physical activity; alcohol consumption in the past 12 months; family history of high blood pressure, stroke, or heart disease; and education).
Hazards ratio corresponds to 5‐ng/dL increase of serum aldosterone concentration.
Comparing a model that included all confounders and smoking status with models that included all confounders, smoking status, and each potential mediator of incident stroke, current smoking was associated with an HR of 2.70 (95% CI 1.71 to 4.25) for total stroke, and the HRs decreased to 2.48 (95% CI 1.53 to 4.01) and 2.49 (95% CI 1.57 to 3.96) after adjusting for FEV1 and aldosterone. When we adjusted for both mediators, the HR for total stroke decreased to an even lower value of 2.32 (95% CI 1.42 to 3.79). Consequently, introduction of FEV1, serum aldosterone, and both mediators reduced the HR of current smoking on incidence of total stroke by 13.1%, 12.1%, and 22.4%, respectively (Table 4). We did not find past smoking to be a significant predictor for total stroke.
Table 4.
FEV1 and Aldosterone as Potential Mediators of the Relationship Between Smoking and Incident Stroke Among Jackson Heart Study Participants (N=5010)
| Mediators in the Multivariate Model | Past Smokers | Current Smokers | |||||
|---|---|---|---|---|---|---|---|
| HRa | 95% CI | P Value | HRa | 95% CI | P Value | Proportion Mediated, % | |
| Total JHS population (156 events, N=5010) | |||||||
| None | 1.16 | 0.76 to 1.77 | 0.486 | 2.70 | 1.71 to 4.25 | <0.001 | — |
| FEV1, L | 1.21 | 0.78 to 1.86 | 0.399 | 2.48 | 1.53 to 4.01 | <0.001 | 13.1 |
| Serum aldosterone, ng/dL | 1.12 | 0.74 to 1.70 | 0.594 | 2.49 | 1.57 to 3.96 | <0.001 | 12.1 |
| FEV1, L and serum aldosterone, ng/dL | 1.16 | 0.75 to 1.80 | 0.498 | 2.32 | 1.42 to 3.79 | <0.001 | 22.4 |
FEV1 indicates forced expiratory volume in the first second; HR, hazard ratio; JHS, Jackson Heart Study.
HR for past smokers and current smokers, respectively, from the Cox models after adjusting for smoking status, other confounders (age; sex; physical activity; alcohol consumption in the past 12 months; family history of high blood pressure, stroke, or heart disease; and education), and different mediators.
Finally, the HRs for FEV1 increased 10.3% (0.61 versus 0.65) after including the systemic inflammatory marker C‐reactive protein (Figure 2A), and the HRs for aldosterone were almost identical for the models that included all confounders and smoking status with or without different blood pressure measurements (Figure 2B).
Figure 2.
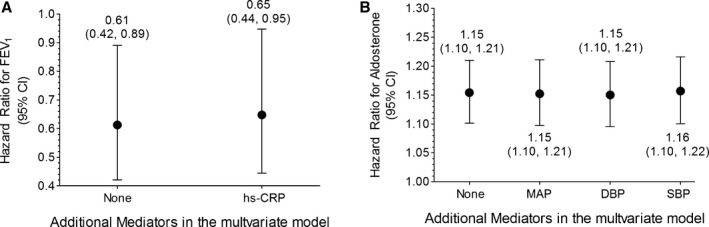
Hazard ratios of per 1‐L increase of FEV 1 (A) and per 5‐ng/dL increase of serum aldosterone (B) for total stroke. All hazard ratios were also adjusted for smoking status, baseline confounders (age; sex; physical activity; alcohol consumption in the past 12 months; family history of high blood pressure, stroke, or heart disease; and education) with or without hs‐CRP for FEV 1 (A) and different blood pressure measurements for aldosterone (B). DBP indicates diastolic blood pressure; FEV1, forced expiratory volume in the first second; hs‐CRP, high‐sensitivity C‐reactive protein; MAP, mean arterial pressure; SBP, systolic blood pressure.
Discussion
In this study, we evaluated the roles of FEV1 and aldosterone as mediators of incident stroke disparity between current and never smokers and quantified the extent to which each of these variables mediated the smoking–stroke relationship in African Americans. Furthermore, we showed that the association between smoking and incident stroke is partially mediated by reduced lung function, in part, through an inflammatory pathway and by aldosterone that is blood pressure independent. Considering that the magnitude of association between smoking and stroke varies across different ethnicities and populations,10 we should be cautious in generalizing our findings to other racial groups. Of note, the vast majority of strokes in our JHS cohort (91.7%, 143 of 156) were ischemic, and when we repeated our analysis using ischemic stroke events only, similar conclusions could be drawn (data not shown).
We also explored the roles of other risk factors for stroke including body mass index, hypertension, diabetes mellitus, total cholesterol, and triglyceride as potential mediators for the smoking–stroke relationship. The reason we considered these stroke risk factors as potential mediators but not as confounders is that they can be affected by smoking exposure and may be located in the causal pathway between smoking and stroke. The change of HRs for current smoking was negligible after we added these variables into the models that included all confounders and smoking status (Table S1), suggesting that no obvious mediation effects through these risk factors were detected using our JHS data. We failed to detect the cross‐sectional association between smoking and diabetes mellitus in the JHS population, although the association between active smoking and the incidence of type 2 diabetes was indicated by a meta‐analysis with 1.2 million participants.41 The association of blood cholesterol with the risk of stroke appears to be under debate, and we did not find any association between baseline total cholesterol and subsequent incident stroke after adjustment for the covariates. Olsen et al reported that higher total serum cholesterol levels are associated with less severe strokes42; however, in the Framingham Heart Study, the risk of carotid stenosis (a precursor of ischemic stroke) was significantly associated with high total serum cholesterol levels.43 Similarly, we did not find any positive association between elevated triglyceride levels and increased risk of stroke, consistent with several other observational studies.44, 45 In addition, the conclusion that FEV1 and aldosterone are mediators of smoking effect on incident stroke is robust after adjusting for these additional risk factors (body mass index, hypertension, diabetes mellitus, total cholesterol, and triglyceride) (Table S2).
Cigarette smoking is associated with a decrease in lower respiratory tract neutrophil elastase, increasing the vulnerability of the lung to elastolytic destruction and thus increasing the risk of development of emphysema.46 Reduced lung function is associated with a significant systemic inflammatory response with increased circulating levels of acute‐phase proteins (ie, C‐reactive protein and fibrinogen), stimulation of the bone marrow with elevated white blood cells and band cells counts, and increased circulating levels of cytokines (ie, interleukin‐1β, tumor necrosis factor‐α, and interleukin‐6). Consequently, the vascular endothelium is activated—an important step in the initiation and progression of atherosclerosis and the destabilization of existing atherosclerotic plaque.47 Due to the cross‐sectional nature of our exposure and mediator variables, we could not determine the temporal sequence between systemic inflammation and reduced lung function. Nonetheless, we advocated and hypothesized that systemic inflammation mediates the association between reduced lung function and cardiovascular disease for the following 2 reasons. First, the association between FEV1 and systemic inflammation was present in those who had never smoked and who did not have asthma or other health problems,48 supporting a direct relationship between FEV1 and systemic inflammation. Second, Hancox et al reported that C‐reactive protein at age 26 years was not a significant predictor of the change in FEV1 between the ages of 26 and 32 years, adjusting for sex and height; in contrast, a fall in FEV1 between the ages of 26 and 32 years was a significant predictor of blood C‐reactive protein levels at age 32 years.18
Smoking has been shown to acutely increase plasma aldosterone, and chronic smoking also raises plasma aldosterone levels.24 Our results indicate that the aldosterone effect on total stroke risk is independent of blood pressure (Figure 2B and Figure S2) and that aldosterone, but neither renin nor the aldosterone:renin ratio, mediates that relationship (n=2214) (Table S3). This result is consistent with previous reports indicating that excess aldosterone is associated with injury in the heart, brain, and kidneys, independent of blood pressure level, and that pharmacological antagonists of aldosterone markedly reduce myocardial injury, cerebral hemorrhage, and renal vascular disease.28, 49, 50 A possible explanation for our findings is that aldosterone may be associated with endothelial dysfunction independent of blood pressure, leading to vascular dysfunction and increased risk of stroke.50
This study has some limitations. First, we used a simplified causal diagram and mediation analysis method adapted from Lu et al,37 and although we consistently adjusted for age, sex, physical activity, alcohol consumption, family history of related diseases, and education as the potential confounders, our results still might be affected by unmeasured and residual confounding. If some of the mediator–outcome confounders are unmeasured or missing, estimates of the direct effect (HR for current smoking when smoking status, mediator, and adjusted confounders are included in the model) might be invalid.51 Second, our analysis did not allow for interactions that might exist between smoking status and mediators.52, 53 Third, we did not have information on the changes in potential mediators (FEV1 and aldosterone) over time and used only baseline measurements in our analysis. Finally, the analysis results in this study cannot be extended to hemorrhagic stroke because of small event numbers in our JHS cohort.
In summary, the current study demonstrates that the association between smoking and total stroke in African Americans is partially mediated through pathways involving lung function and aldosterone. Further work is warranted to examine alternative mechanisms implicated in the smoking–stroke association.
Sources of Funding
The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities.
Disclosures
None.
Supporting information
Data S1. Statistical Analysis (three‐path mediation effects estimation using linear structural equation modeling framework).
Table S1. Hazard Ratios and Excess Risk of Smoking Status Through Different Risk Factors Among Total Jackson Heart Study Participants (N=5010)
Table S2. Forced Expiratory Volume in the First Second and Aldosterone as Potential Mediators of the Relationship Between Smoking and Incident Stroke After Adjusting for Additional Risk Factors Among Jackson Heart Study Participants (N=5010)
Table S3. Aldosterone But Not Renin or Aldosterone:Renin Ratio as a Potential Mediator of the Relationship Between Smoking and Incident Stroke Among the Jackson Heart Study Subpopulation With Renin Activity Measurement (N=2214)
Figure S1. Estimation of the potential mediating effects of forced expiratory volume in the first second and high‐sensitivity C‐reactive protein on the relationship between smoking and incident stroke using the linear structural equation modeling approach. Estimated β‐coefficients with the standard errors for the estimates (in parentheses) are displayed. The dotted arrow represents the direct effect, the dashed arrow represents the indirect effect, and the solid arrows represent sources of noncausal association of multiple confounders with the potential mediators and the outcome.
Figure S2. Estimation of the potential mediating effects of aldosterone and blood pressure (mean arterial pressure) on the relationship between smoking and incident stroke using the linear structural equation modeling approach. Estimated β–coefficients with the standard errors for the estimates (in parentheses) are displayed. The dotted arrow represents the direct effect, the dashed arrow represents the indirect effect, and the solid arrows represent sources of noncausal association of multiple confounders with the potential mediators and the outcome.
(J Am Heart Assoc. 2016;5:e002689 doi: 10.1161/JAHA.115.002689)
Accompanying Data S1, Tables S1 through S3, and Figures S1 and S2 are available at http://jaha.ahajournals.org/content/5/1/e002689/suppl/DC1
References
- 1. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. [DOI] [PubMed] [Google Scholar]
- 2. Brain basics: preventing stroke. National Institute of Neurological Disorders and Stroke web site. Available at: http://www.ninds.nih.gov/disorders/stroke/preventing_stroke.htm. Accessed February 10, 2015.
- 3. Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, Goldstein LB, Gorelick PB, Howard G, Kittner SJ, Manolio TA, Whisnant JP, Wolf PA. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk factors. Stroke. 1997;28:1507–1517. [DOI] [PubMed] [Google Scholar]
- 4. Rundek T, Sacco RL. Risk factor management to prevent first stroke. Neurol Clin. 2008;26:1007–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asplund K, Karvanen J, Giampaoli S, Jousilahti P, Niemelä M, Broda G, Cesana G, Dallongeville J, Ducimetriere P, Evans A, Ferrières J, Haas B, Jorgensen T, Tamosiunas A, Vanuzzo D, Wiklund PG, Yarnell J, Kuulasmaa K, Kulathinal S; MORGAM Project . Relative risks for stroke by age, sex, and population based on follow‐up of 18 European populations in the MORGAM Project. Stroke. 2009;40:2319–2326. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y, Galloway JM, Welty TK, Wiebers DO, Whisnant JP, Devereux RB, Kizer JR, Howard BV, Cowan LD, Yeh J, Howard WJ, Wang W, Best L, Lee ET. Incidence and risk factors for stroke in American Indians: the Strong Heart Study. Circulation. 2008;118:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dagenais GR, Yi Q, Lonn E, Sleight P, Ostergren J, Yusuf S; HOPE Trial Investigators . Impact of cigarette smoking in high‐risk patients participating in a clinical trial. A substudy from the Heart Outcomes Prevention Evaluation (HOPE) trial. Eur J Cardiovasc Prev Rehabil. 2005;12:75–81. [PubMed] [Google Scholar]
- 8. Yamagishi K, Iso H, Kitamura A, Sankai T, Tanigawa T, Naito Y, Sato S, Imano H, Ohira T, Shimamoto T. Smoking raises the risk of total and ischemic strokes in hypertensive men. Hypertens Res. 2003;26:209–217. [DOI] [PubMed] [Google Scholar]
- 9. Abbott RD, Yin Y, Reed DM, Yano K. Risk of stroke in male cigarette smokers. N Engl J Med. 1986;315:717–720. [DOI] [PubMed] [Google Scholar]
- 10. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Omori H, Nonami Y, Morimoto Y. Effect of smoking on FEV decline in a cross‐sectional and longitudinal study of a large cohort of Japanese males. Respirology. 2005;10:464–469. [DOI] [PubMed] [Google Scholar]
- 12. Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS, Tashkin DP; Lung Health Study Research Group . Smoking cessation and lung function in mild‐to‐moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161:381–390. [DOI] [PubMed] [Google Scholar]
- 13. Campbell Jenkins BW, Sarpong DF, Addison C, White MS, Hickson DA, White W, Burchfiel C. Joint effects of smoking and sedentary lifestyle on lung function in African Americans: the Jackson Heart Study cohort. Int J Environ Res Public Health. 2014;11:1500–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee PN, Fry JS. Systematic review of the evidence relating FEV1 decline to giving up smoking. BMC Med. 2010;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hozawa A, Billings JL, Shahar E, Ohira T, Rosamond WD, Folsom AR. Lung function and ischemic stroke incidence: the Atherosclerosis Risk in Communities Study. Chest. 2006;130:1642–1649. [DOI] [PubMed] [Google Scholar]
- 16. Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond WD, Heiss G. Lung function and incident coronary heart disease: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158:1171–1181. [DOI] [PubMed] [Google Scholar]
- 17. Truelsen T, Prescott E, Lange P, Schnohr P, Boysen G. Lung function and risk of fatal and non‐fatal stroke. The Copenhagen City Heart Study. Int J Epidemiol. 2001;30:145–151. [DOI] [PubMed] [Google Scholar]
- 18. Hancox RJ, Poulton R, Greene JM, Filsell S, McLachlan CR, Rasmussen F, Taylor DR, Williams MJ, Williamson A, Sears MR. Systemic inflammation and lung function in young adults. Thorax. 2007;62:1064–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gan WQ, Man SF, Sin DD. The interactions between cigarette smoking and reduced lung function on systemic inflammation. Chest. 2005;127:558–564. [DOI] [PubMed] [Google Scholar]
- 20. Kool MJ, Hoeks AP, Struijker Boudier HA, Reneman RS, Van Bortel LM. Short‐ and long‐term effects of smoking on arterial wall properties in habitual smokers. J Am Coll Cardiol. 1993;22:1881–1886. [DOI] [PubMed] [Google Scholar]
- 21. Mast H, Thompson JL, Lin IF, Hofmeister C, Hartmann A, Marx P, Mohr JP, Sacco RL. Cigarette smoking as a determinant of high‐grade carotid artery stenosis in Hispanic, black, and white patients with stroke or transient ischemic attack. Stroke. 1998;29:908–912. [DOI] [PubMed] [Google Scholar]
- 22. Hioki H, Aoki N, Kawano K, Homori M, Hasumura Y, Yasumura T, Maki A, Yoshino H, Yanagisawa A, Ishikawa K. Acute effects of cigarette smoking on platelet‐dependent thrombin generation. Eur Heart J. 2001;22:56–61. [DOI] [PubMed] [Google Scholar]
- 23. Zhu BQ, Parmley WW. Hemodynamic and vascular effects of active and passive smoking. Am Heart J. 1995;130:1270–1275. [DOI] [PubMed] [Google Scholar]
- 24. Baer L, Radichevich I. Cigarette smoking in hypertensive patients. Blood pressure and endocrine responses. Am J Med. 1985;78:564–568. [DOI] [PubMed] [Google Scholar]
- 25. Laustiola KE, Lassila R, Nurmi AK. Enhanced activation of the renin‐angiotensin‐aldosterone system in chronic cigarette smokers: a study of monozygotic twin pairs discordant for smoking. Clin Pharmacol Ther. 1988;44:426–430. [DOI] [PubMed] [Google Scholar]
- 26. Freel EM, Connell JM. Mechanisms of hypertension: the expanding role of aldosterone. J Am Soc Nephrol. 2004;15:1993–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Satoh M, Kikuya M, Ohkubo T, Mori T, Metoki H, Hara A, Utsugi MT, Hashimoto T, Hirose T, Obara T, Inoue R, Asayama K, Kanno A, Totsune K, Hoshi H, Satoh H, Imai Y. Aldosterone‐to‐renin ratio as a predictor of stroke under conditions of high sodium intake: the Ohasama study. Am J Hypertens. 2012;25:777–783. [DOI] [PubMed] [Google Scholar]
- 28. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. [DOI] [PubMed] [Google Scholar]
- 29. Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6‐4‐17. [PubMed] [Google Scholar]
- 30. Wyatt SB, Diekelmann N, Henderson F, Andrew ME, Billingsley G, Felder SH, Fuqua S, Jackson PB. A community‐driven model of research participation: the Jackson Heart Study Participant Recruitment and Retention Study. Ethn Dis. 2003;13:438–455. [PubMed] [Google Scholar]
- 31. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 32. Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 33. Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. [DOI] [PubMed] [Google Scholar]
- 34. Ira S. Human Physiology. 5th ed Iowa: William C Brown Publishers; 1996. [Google Scholar]
- 35. Keku E, Rosamond W, Taylor HA Jr, Garrison R, Wyatt SB, Richard M, Jenkins B, Reeves L, Sarpong D. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis. 2005;15:S6‐62‐70. [PubMed] [Google Scholar]
- 36. Lange T, Hansen JV. Direct and indirect effects in a survival context. Epidemiology. 2011;22:575–581. [DOI] [PubMed] [Google Scholar]
- 37. Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body‐mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baron RM, Kenny DA. The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 39. Taylor AB, MacKinnon D, Tein JY. Test of the three‐path mediated effect. Organ Res Methods. 2008;11:241–269. [Google Scholar]
- 40. Ditlevsen S, Christensen U, Lynch J, Damsgaard MT, Keiding N. The mediation proportion: a structural equation approach for estimating the proportion of exposure effect on outcome explained by an intermediate variable. Epidemiology. 2005;16:114–120. [DOI] [PubMed] [Google Scholar]
- 41. Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta‐analysis. JAMA. 2007;298:2654–2664. [DOI] [PubMed] [Google Scholar]
- 42. Olsen TS, Christensen RH, Kammersgaard LP, Andersen KK. Higher total serum cholesterol levels are associated with less severe strokes and lower all‐cause mortality: ten‐year follow‐up of ischemic strokes in the Copenhagen Stroke Study. Stroke. 2007;38:2646–2651. [DOI] [PubMed] [Google Scholar]
- 43. Wilson PW, Hoeg JM, D'Agostino RB, Silbershatz H, Belanger AM, Poehlmann H, O'Leary D, Wolf PA. Cumulative effects of high cholesterol levels, high blood pressure, and cigarette smoking on carotid stenosis. N Engl J Med. 1997;337:516–522. [DOI] [PubMed] [Google Scholar]
- 44. Shahar E, Chambless LE, Rosamond WD, Boland LL, Ballantyne CM, McGovern PG, Sharrett AR; Atherosclerosis Risk in Communities Study . Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2003;34:623–631. [DOI] [PubMed] [Google Scholar]
- 45. Wannamethee SG, Shaper AG, Ebrahim S. HDL‐cholesterol, total cholesterol, and the risk of stroke in middle‐aged British men. Stroke. 2000;31:1882–1888. [DOI] [PubMed] [Google Scholar]
- 46. Ogushi F, Hubbard RC, Vogelmeier C, Fells GA, Crystal RG. Risk factors for emphysema. Cigarette smoking is associated with a reduction in the association rate constant of lung alpha 1‐antitrypsin for neutrophil elastase. J Clin Invest. 1991;87:1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tamagawa E, van Eeden SF. Impaired lung function and risk for stroke: role of the systemic inflammation response? Chest. 2006;130:1631–1633. [DOI] [PubMed] [Google Scholar]
- 48. Aronson D, Roterman I, Yigla M, Kerner A, Avizohar O, Sella R, Bartha P, Levy Y, Markiewicz W. Inverse association between pulmonary function and C‐reactive protein in apparently healthy subjects. Am J Respir Crit Care Med. 2006;174:626–632. [DOI] [PubMed] [Google Scholar]
- 49. Takeda R, Matsubara T, Miyamori I, Hatakeyama H, Morise T. Vascular complications in patients with aldosterone producing adenoma in Japan: comparative study with essential hypertension. The Research Committee of Disorders of Adrenal Hormones in Japan. J Endocrinol Invest. 1995;18:370–373. [DOI] [PubMed] [Google Scholar]
- 50. Rocha R, Stier CT Jr. Pathophysiological effects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab. 2001;12:308–314. [DOI] [PubMed] [Google Scholar]
- 51. Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013;42:1511–1519. [DOI] [PubMed] [Google Scholar]
- 52. Hata J, Doi Y, Ninomiya T, Fukuhara M, Ikeda F, Mukai N, Hirakawa Y, Kitazono T, Kiyohara Y. Combined effects of smoking and hypercholesterolemia on the risk of stroke and coronary heart disease in Japanese: the Hisayama study. Cerebrovasc Dis. 2011;31:477–484. [DOI] [PubMed] [Google Scholar]
- 53. Hashimoto T, Kikuya M, Ohkubo T, Satoh M, Metoki H, Inoue R, Asayama K, Kanno A, Obara T, Hirose T, Hara A, Hoshi H, Totsune K, Satoh H, Sato H, Imai Y. Home blood pressure level, blood pressure variability, smoking, and stroke risk in Japanese men: the Ohasama study. Am J Hypertens. 2012;25:883–891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Statistical Analysis (three‐path mediation effects estimation using linear structural equation modeling framework).
Table S1. Hazard Ratios and Excess Risk of Smoking Status Through Different Risk Factors Among Total Jackson Heart Study Participants (N=5010)
Table S2. Forced Expiratory Volume in the First Second and Aldosterone as Potential Mediators of the Relationship Between Smoking and Incident Stroke After Adjusting for Additional Risk Factors Among Jackson Heart Study Participants (N=5010)
Table S3. Aldosterone But Not Renin or Aldosterone:Renin Ratio as a Potential Mediator of the Relationship Between Smoking and Incident Stroke Among the Jackson Heart Study Subpopulation With Renin Activity Measurement (N=2214)
Figure S1. Estimation of the potential mediating effects of forced expiratory volume in the first second and high‐sensitivity C‐reactive protein on the relationship between smoking and incident stroke using the linear structural equation modeling approach. Estimated β‐coefficients with the standard errors for the estimates (in parentheses) are displayed. The dotted arrow represents the direct effect, the dashed arrow represents the indirect effect, and the solid arrows represent sources of noncausal association of multiple confounders with the potential mediators and the outcome.
Figure S2. Estimation of the potential mediating effects of aldosterone and blood pressure (mean arterial pressure) on the relationship between smoking and incident stroke using the linear structural equation modeling approach. Estimated β–coefficients with the standard errors for the estimates (in parentheses) are displayed. The dotted arrow represents the direct effect, the dashed arrow represents the indirect effect, and the solid arrows represent sources of noncausal association of multiple confounders with the potential mediators and the outcome.


