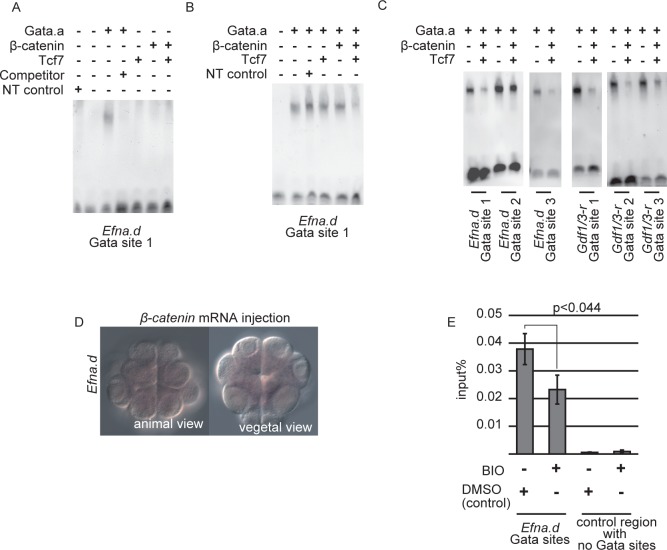
Fig 7. Gata.a binding activity is suppressed in a ternary complex with Tcf7 and β-catenin.
(A) Gata.a protein produced in vitro specifically recognized the Gata site critical for Efna.d expression. As a negative control, we used a rabbit reticulocyte lysate that did not contain template plasmids (NT control). (B) Co-incubation of Gata.a with either β-catenin or Tcf7 did not affect the binding activity of Gata.a for the proximal Gata site (Gata site 1) in the upstream sequence of Efna.d, whereas co-incubation of Gata.a with β-catenin and Tcf7 reduced the binding activity of Gata.a. (C) Co-incubation of Gata.a with β-catenin and Tcf7 reduced the binding activity of Gata.a for Gata sites in the upstream sequences of Efna.d and Gdf1/3-r. (D) Expression of Efna.d was suppressed in embryos injected with β-catenin mRNA. (E) ChIP followed by quantitative PCR revealed that BIO treatment reduced Gata-a binding to the Efna.d upstream region. Error bars indicate standard errors of three independent experiments.