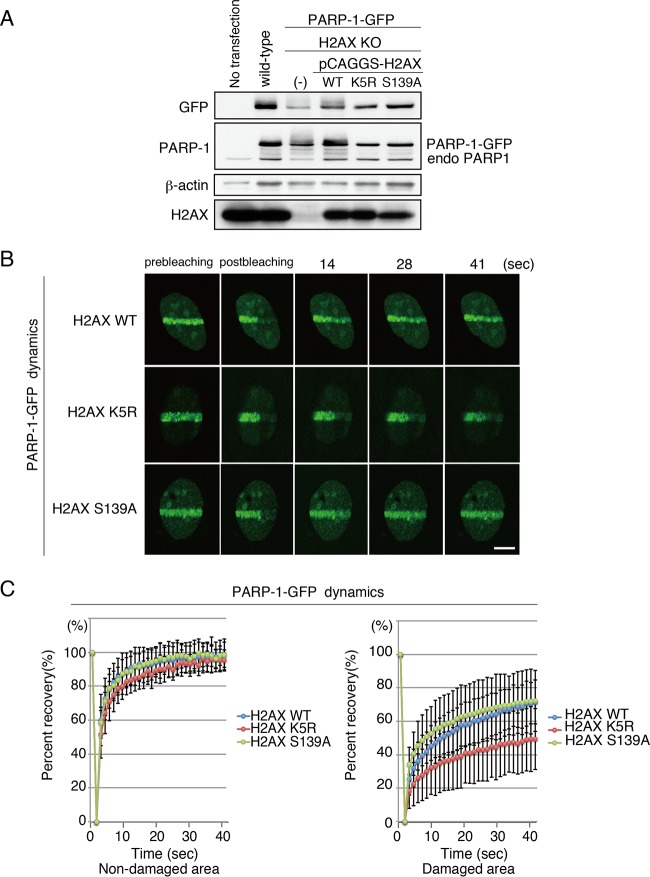
FIG 6.
K5 acetylation, but not S139 phosphorylation, of H2AX is required for dynamic binding of PARP-1 to DNA damage sites. (A) Immunoblot analysis of H2AX KO MEF cells and that reconstituted with either the WT, K5R, or S139A version of H2AX. PARP-1–GFP also was stably expressed. Anti-GFP, anti-PARP-1, anti-beta-actin, and anti-H2AX were used. (B) FRAP analysis to monitor the dynamics of PARP-1–GFP. PARP-1–GFP accumulated at the microirradiated area was photobleached. H2AX KO MEF cells that were reconstituted with either H2AX WT, H2AX K5R, or H2AX S139A were used. Bar, 10 μm. (C) Fluorescence recovery of the GFP signal of PARP-1–GFP in either the nondamaged (left; H2AX WT, 98.7% ± 9.1% [n = 15]; H2AX K5R, 95.2% ± 6.2% [n = 13]; H2AX S139A, 98.4% ± 7.4% [n = 16]) or damaged area (right; H2AX WT, 71.4% ± 13.0% [n = 14]; H2AX K5R, 49.4% ± 18.3% [n = 14]; H2AX S139A, 72.1% ± 12.6% [n = 16]; P value of 0.88 between H2AX WT- and H2AX S139A-expressing cells and P value of 0.00042 between H2AX K5R- and H2AX S139A-expressing cells) was quantified and plotted.