ABSTRACT
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract disease, which causes high rates of morbidity and mortality in infants and the elderly. Models of human RSV pulmonary disease are needed to better understand RSV pathogenesis and to assess the efficacy of RSV vaccines. We assessed the RSV-specific human innate, humoral, and cellular immune responses in humanized mice (mice with a human immune system [HIS mice]) with functional human CD4+ T and B cells. These mice were generated by introduction of HLA class II genes, various human cytokines, and human B cell activation factor into immunodeficient NOD scid gamma (NSG) mice by the use of an adeno-associated virus vector, followed by engraftment of human hematopoietic stem cells. During the first 3 days of infection, HIS mice lost more weight and cleared RSV faster than NSG mice. Human chemokine (C-C motif) ligand 3 (CCL3) and human interleukin-1β (IL-1β) expression was detected in the RSV-infected HIS mice. The pathological features induced by RSV infection in HIS mice included peribronchiolar inflammation, neutrophil predominance in the bronchioalveolar lavage fluid, and enhanced airway mucus production. Human anti-RSV IgG and RSV-neutralizing antibodies were detected in serum and human anti-RSV mucosal IgA was detected in bronchioalveolar lavage fluid for up to 6 weeks. RSV infection induced an RSV-specific human gamma interferon response in HIS mouse splenocytes. These results indicate that human immune cells can induce features of RSV lung disease, including mucus hyperplasia, in murine lungs and that HIS mice can be used to elicit human anti-RSV humoral and cellular immunity.
IMPORTANCE Infections with respiratory syncytial virus (RSV) are common and can cause severe lung disease in infants and the elderly. The lack of a suitable animal model with disease features similar to those in humans has hampered efforts to predict the efficacy of novel anti-RSV therapies and vaccines for use in humans. A murine model consisting of mice with a human immune system (HIS mice) could be useful for assessment of RSV disease and anti-RSV responses specific to humans. This study investigates an HIS mouse model to imitate human RSV disease and immune responses. We found that RSV lung infection in HIS mice results in an RSV-specific pathology that mimics RSV disease in humans and induces human anti-RSV immune responses. This model could be useful for better understanding of human RSV disease and for the development of RSV therapies.
INTRODUCTION
Infection of the lower respiratory tract with respiratory syncytial virus (RSV) is the most common cause for hospitalization of infants and children (1) and globally causes up to 200,000 deaths in children under the age of 5 years (2). Premature infants, especially those with chronic lung disease or congenital heart disease (3), and the elderly (4) are the most susceptible to the development of severe disease. Early RSV infections are also associated with the later development of asthma (5). No efficient therapeutics or vaccines active against RSV are available. Only immunoprophylaxis with palivizumab, a monoclonal anti-RSV F antibody, provides some protection for infants at risk (6). Several animal models have been developed to model human RSV disease (7). As mouse models have limitations in mimicking human RSV disease, better models would be useful for the preclinical assessment of novel anti-RSV therapies and vaccines. Humoral immunity is essential in the prevention of RSV infections. Higher levels of maternally derived antibodies (8) and prophylactic administration of intravenous immunoglobulin enriched for high levels of RSV-neutralizing antibodies (9) or humanized monoclonal antibody against RSV (10) are associated with a reduction of disease severity in RSV-infected infants. Therefore, a humanized mouse model with functional human CD4+ T and B cells would be useful to assess the contributions of these immune cells to the lung disease induced by RSV infection.
Murine models consisting of mice with a human immune system (HIS mice) have been developed to study the mechanisms of infection and human immune responses against human pathogens and to test the efficacy of vaccines (11–14). We recently established HIS mice that possess functional human CD8+ or CD4+ T and B cells. These mice were generated by the introduction of an adeno-associated virus serotype 9 (AAV9) vector carrying human cytokine genes into highly immunodeficient NOD scid gamma (NSG) mice followed by engraftment of human hematopoietic stem cells (15, 16). In this study, we investigated the use of HIS mice with human CD4+ T cells and B cells as a model for human RSV disease with the following two objectives: (i) to assess if this model leads to features of human RSV lung disease, even those that are not primarily thought to be associated with immune cell function, such as mucus cell hyperplasia, and (ii) to evaluate if this model can be used to generate human neutralizing antibodies, one of the major immune correlates for protection. We found that the RSV-specific pathology generated in these mice mimics the RSV disease in humans and, importantly, that these mice induce human anti-RSV immune responses, including human neutralizing antibodies. These findings further highlight the role of human immune cells in pulmonary RSV pathogenesis.
MATERIALS AND METHODS
RSV.
The RSV strain Line 19 was propagated, purified, and quantified as described previously (17).
Mice.
NSG (NOD.Cg-Prkdcscid IL2rgtmWjl/Sz) mice were purchased from The Jackson Laboratory and maintained under specific-pathogen-free conditions in the animal facilities at the Comparative Bioscience Center of The Rockefeller University. All animal experiments were carried out in strict accordance with the policy on the humane care and use of laboratory animals of the United States Public Health Service (18). The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at The Rockefeller University (assurance number A3081-01). HIS mice that possess functional human CD4+ T and B cells through AAV9-mediated modification with human cytokine genes were used in this study (16). To obtain HIS mice, young NSG mice (2 to 3 weeks old) were first inoculated with 1 × 1011 genomic copies (GC) of AAV9-HLA class II (DR1 or DR4) (5 × 1010 GC intrathoracically and 5 × 1010 GC intraperitoneally), together with intraperitoneal injection of 5 × 1010 GC of AAV9–B cell-activating factor belonging to the tumor necrosis factor family (BAFF) and 5 × 109 GC of AAV9-human cytokines (AAV9–interleukin-3 [IL-3], AAV9–IL-4, AAV9–IL-7, AAV9–IL-15, AAV9–granulocyte macrophage colony-stimulating factor, and AAV9–macrophage colony-stimulating factor). Two weeks later, the mice were subjected to sublethal irradiation, followed by intravenous administration of 1 × 105 human CD34+ hematopoietic stem cells (HSCs) derived from human fetal liver (Advanced Bioscience Resources, Alameda, CA). The levels of human peripheral blood mononuclear cells (PBMCs) in the mouse peripheral blood are shown in Table 1. Since these HIS mice received only HLA class II and not HLA class I, the human CD8 cells in these mice are nonfunctional. NSG mice not infected with the AAV9 vector or transfused with human HSCs were used as negative controls.
TABLE 1.
Human leukocyte reconstitution in the peripheral blood of NSG mice transduced with AAV9-DR1/DR4, AAV9-human BAFF, and AAV9-human cytokinesa
| HIS mouse identifier | % cells |
|||||
|---|---|---|---|---|---|---|
| Human PBMCs | CD3 human T cells | CD8 human T cells | CD4 human T cells | CD19 human B cells | CD161+ CD3− human NK cells | |
| 57b | 94.5 | 18.5 | 22.8 | 73.6 | 48 | 25.8 |
| 61b | 86.6 | 29.4 | 30.2 | 67.3 | 49.1 | 11.7 |
| 584b | 70 | 75.4 | 35.6 | 63.9 | 15 | 6.5 |
| 336b | 86.7 | 74.3 | 50 | 49.3 | 15.9 | 5 |
| 585b | 83.8 | 61.9 | 37 | 62.2 | 22.3 | 8.8 |
| 600b | 78.7 | 90.7 | 33.2 | 66.5 | 1 | 3.4 |
| 302b | 85.8 | 74 | 18.7 | 80.9 | 19.1 | 2.5 |
| 330b | 64.2 | 54.3 | 15.8 | 83.4 | 32.6 | 5.6 |
| 700b | 58.9 | 52 | 21.6 | 78.3 | 38.5 | 3.4 |
| 830 | 81.5 | 20.2 | 29.7 | 50.2 | 49 | 19.2 |
| 837 | 87.9 | 7.3 | 3.1 | 7.9 | 49.8 | 16.4 |
| 842 | 32.5 | 30.9 | 36.5 | 45.6 | 28.6 | 6 |
| 846 | 65.9 | 30.9 | 42.5 | 50.9 | 49.8 | 13.2 |
| 847 | 3.1 | 6.4 | 20 | 80 | 7.7 | 7.7 |
| 849 | 11.8 | 69 | 52.3 | 45 | 4.4 | 2.5 |
| 141 | 18.9 | 4.5 | 33.3 | 43.3 | 35.5 | 4.6 |
| 145 | 8.2 | 17.1 | 55.9 | 26.5 | 25.1 | 9 |
| 831 | 59 | 19.1 | 21.8 | 30 | 47.9 | 13.4 |
| 845 | 12.6 | 4.1 | 23.5 | 41.2 | 56.4 | 20.2 |
| 668 | 80.4 | 87 | 26.2 | 72.1 | 1.3 | 8.9 |
| 669 | 79 | 74.9 | 48.5 | 48.8 | 2 | 18.1 |
| 672 | 81.6 | 67.3 | 42.3 | 56.4 | 5.4 | 20.9 |
| 682 | 98 | 68.9 | 42 | 56.9 | 10.1 | 17.5 |
| 628 | 44.7 | 31.6 | 26.3 | 71.3 | 1.4 | 55.9 |
| 629 | 83.1 | 0.3 | 58.8 | 0 | 1.7 | 83.5 |
| 681 | 10.1 | 0 | 0 | 0 | 8.8 | 33.3 |
The level of human CD45+ cell reconstitution in the blood was determined using flow cytometry at 15 weeks after human CD34+ cells were engrafted into NSG mice transduced with an AAV9 vector carrying HLA-DR1/DR4, human BAFF, or selected human cytokines. Shown are the percentages of human CD3+ T cells, human CD4+ T cells, and CD19+ B cells within human CD45+ cells in the blood of various HIS mice.
HIS mice utilized to study short-term responses to RSV infection.
Rapid host responses to intranasal challenge with RSV.
To evaluate the host responses against RSV pulmonary infection, HIS and NSG mice were challenged with RSV Line 19 (106 PFU in 50 μl phosphate-buffered saline [PBS] per mouse) by intranasal inoculation. Three days later the mice were sacrificed, and lung homogenates were stored at −80°C or in TRIzol reagent (Invitrogen) for RNA isolation. The RSV titers in lung homogenates were quantified by plaque assay and expressed as the number of PFU per lung as previously described (19). RNA was converted to cDNA using random hexamers and reverse transcription (RT) reagents (Applied Biosystems). Quantification of the relative levels of expression of mRNA for murine and human IL-1β, murine and human chemokine (C-C motif) ligand 3 (CCL3), and the murine mucus gene Muc5ac was performed utilizing TaqMan gene expression assays (catalog numbers Mm00441259_g1, Mm00434228_m1, Hs04194942_s1, Hs01555410_m1, and Mm01276735_m1; Applied Biosystems). One hundred nanograms of cDNA was processed for quantitative RT-PCR (qRT-PCR) using a specific 6-carboxyfluorescein-labeled TaqMan MGB probe and primers (Applied Biosystems). Endogenous murine GAPDH (glyceraldehyde-3-phosphate dehydrogenase; catalog number 4352932E) was used for normalization of the mRNA levels. qRT-PCR was performed using an ABI Prism 7000 sequence detection system (Applied Biosystems) under conditions of 40 cycles of denaturation (95°C for 15 s) and annealing/extension (60°C for 1 min). The relative quantity of each mRNA was calculated by the ΔΔCT threshold cycle (CT) method. Lung histopathology and differential cell counts in bronchioalveolar lavage (BAL) fluid were evaluated as previously described (17, 19).
Anti-RSV adaptive immune responses.
HIS and NSG mice were infected intranasally with RSV (106 PFU per mouse) and were infected again 1 and 6 weeks later. Serum was collected at 2, 4, and 7 weeks following initial RSV administration. BAL fluid was collected by intratracheal instillation and aspiration of 0.5 ml PBS. Anti-RSV human IgG in serum and human IgA in BAL fluid were assessed by enzyme-linked immunosorbent assay (ELISA; Bio-Rad Laboratories) as previously described, with modifications (17, 19). RSV-neutralizing antibody titers in serum were quantified by plaque assay, as previously described (17, 19).
The RSV-specific cellular immune response was determined by an enzyme-linked immunosorbent spot (ELISPOT) assay for human gamma interferon (IFN-γ) secreted from splenocytes following stimulation with heat-inactivated (56°C, 30 min) RSV. Mouse splenocytes were isolated at 7 weeks as a single-cell suspension and cultured in RPMI medium supplemented with 2% fetal bovine serum (HyClone, Logan, UT), 10 mM HEPES (pH 7.5; Biosource International, Camarillo, CA), and 10 μM β-mercaptoethanol (Sigma-Aldrich) in 96-well MultiScreen-HA plates (Millipore) coated with of 10 μg/ml of anti-human IFN-γ capture antibody (Mabtech). The splenocytes (106/well) were treated with 10 μg/ml of heat-inactivated purified RSV for 24 h. The wells were washed, incubated with a biotinylated anti-human IFN-γ antibody (1 μg/ml; Mabtech) for 2 h followed by avidin-horseradish peroxidase (1:1,000), and developed using angiotensin-converting enzyme (ACE) substrate (BD Biosciences). The number of distinct spots was counted under a stereomicroscope.
Statistical analysis.
Data are presented as the mean ± standard error of the mean (SEM). Statistical analyses were performed using a nonpaired two-tailed Student's t test or two-way analysis of variance, and statistical significance was determined to be a P value of <0.05.
RESULTS
Short-term responses of HIS mice to RSV respiratory infection.
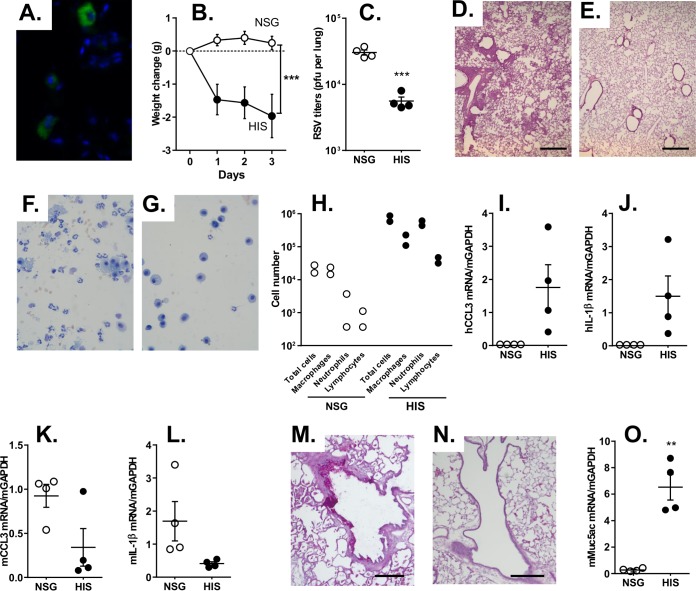
Human CD45+ cells were detected throughout the lungs of HIS mice (Fig. 1A) and were absent in NSG mice (not shown), confirming the presence of human immune cells in the respiratory tract. To assess the susceptibility of the HIS mice to RSV respiratory infection, HIS and NSG control mice were infected with RSV via the intranasal route. Interestingly, only the HIS mice and not the NSG mice showed a loss of body weight on intranasal infection with RSV (P < 0.0001; Fig. 1B), suggesting a stronger host inflammatory response to the infection in the HIS mice. Quantification of the RSV load after 3 days showed a lower level of RSV in the HIS mice than the NSG mice (P < 0.0001; Fig. 1C), indicating the more productive clearance of the virus in the HIS mice. RSV-infected HIS mice showed predominant peribronchiolar inflammatory cell infiltrates, which are characteristic of RSV bronchiolitis (Fig. 1D). Minimal inflammation was present in the RSV-infected NSG mouse controls (Fig. 1E). Likewise, the BAL fluid retrieved from the RSV-infected HIS mice contained more neutrophils and lymphocytes (Fig. 1F), whereas the BAL fluid from RSV-infected NSG mice had a predominance of macrophages (Fig. 1G). Quantification of the BAL fluid cells from RSV-infected HIS mice showed an increase in the numbers of macrophages, neutrophils, and lymphocytes (Fig. 1H), indicative of a neutrophil-predominant inflammatory response that is characteristic for RSV respiratory infection at this time point. To assess if RSV induced a human chemokine/cytokine response that is typically seen with RSV respiratory infections, expression of the chemokine CCL3 and the cytokine IL-1β in lung tissue from infected mice was quantified. Interestingly, human CCL3 and IL-1β were detected in all the HIS mice infected with RSV but not, as expected, in NSG mice (Fig. 1I and J). Murine CCL3 and IL-1β were detected in both RSV-infected NSG mice and RSV-infected HIS mice (Fig. 1K and L). This suggests that RSV infection in the HIS mice induced a robust human cell-derived chemokine/cytokine response, in addition to the murine cell-derived responses. To assess if RSV can elicit a strong mucus response, the mucus-producing cells were visualized by the use of periodic acid-Schiff (PAS) stain, and the expression of the murine mucus gene Muc5ac in the lung was quantified. Numerous PAS-positive airway epithelial cells were seen in the HIS mice compared to those in the NSG mice infected with RSV (Fig. 1M and N). The level of Muc5ac mRNA expression was increased in the RSV-infected HIS mice (P < 0.001; Fig. 1O). Overall, these results indicate that RSV infection in the HIS mice showed characteristic early responses, including the inflammation and mucus induction that is typically seen with RSV respiratory infections.
FIG 1.
Short-term responses of HIS mice to RSV respiratory infection. HIS and NSG mice were infected via the intranasal route with RSV Line 19 (106 PFU/mouse for all mice). (A) Human CD45-positive cells in a lung of an HIS mouse. (B) Body weight change in mice following RSV infection. (C) RSV titers in lung homogenates at 3 days following infection, determined by plaque assay. (D and E) Lung histology (hematoxylin and eosin stain) from RSV-infected HIS (D) or NSG (E) mice at 3 days following infection. Bars = 500 μm. (F and G) Morphology of BAL fluid cells (Giemsa stain) from RSV-infected HIS (F) or NSG (G) mice. (H) Differential quantification of cells in BAL fluid. (I to L) Inflammatory human and murine CCL3 and IL-1β mRNA expression at 3 days following infection, analyzed by relative qRT-PCR, as follows: human CCL3 (I), human IL-1β (J), murine CCL3 (K), and murine IL-1β (L). (M and N) Mucus-secreting cells from HIS (M) or NSG (N) mice visualized with PAS stain. Bars = 100 μm. (O) Relative quantification of murine Muc5ac mRNA by qRT-PCR. Data are shown as the means ± SEMs for 4 to 5 mice per group. **, P < 0.001; ***, P < 0.0001. h, human; m, murine.
RSV induces human humoral and cellular immune responses in HIS mice.
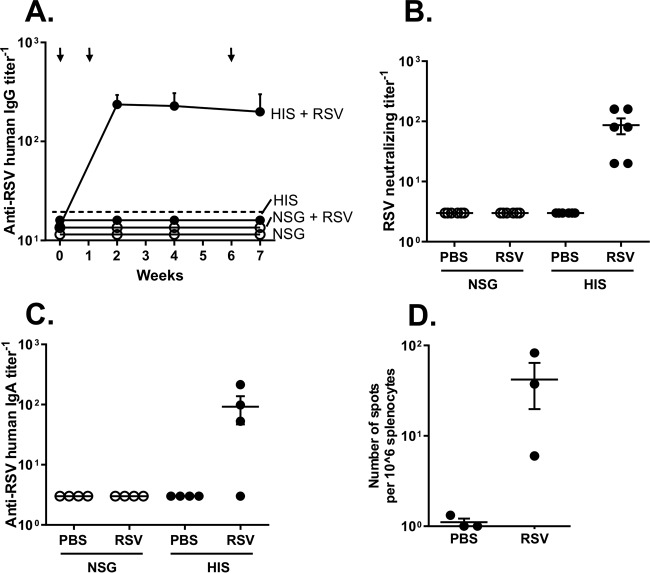
To assess anti-RSV humoral immune responses, RSV-specific human IgG antibodies in RSV-infected or noninfected HIS and NSG mouse sera were analyzed by ELISA for up to 7 weeks. All RSV-infected HIS mice showed anti-RSV serum human IgG at 2, 4, and 7 weeks following infection (Fig. 2A). In contrast, no antibodies were detected in the RSV-infected NSG mice or the uninfected HIS or NSG mice. Importantly, all RSV-infected HIS mice had RSV-neutralizing antibodies (Fig. 2B). To assess the mucosal IgA response, anti-RSV human IgA antibodies in BAL fluid were analyzed at 7 weeks following infection. RSV-infected HIS mice (3 out of 4) had detectable anti-RSV human IgA (Fig. 2C). Importantly, RSV-infected HIS mice had RSV-specific human IFN-γ-positive spleen cells at 7 weeks following infection (Fig. 2D). This suggests that HIS mice are able to mount RSV-specific systemic and mucosal human humoral and cellular immune responses.
FIG 2.
Adaptive humoral and cellular immune responses in HIS mice following RSV respiratory infection. HIS and NSG mice were infected via the intranasal route with RSV Line 19 (106 PFU/mouse for all mice). (A) Anti-RSV human IgG antibody levels in serum at 0, 2, 4, and 7 weeks analyzed by ELISA. The limit of detection is indicated by the dashed line. The arrows indicate the time points of RSV inoculation. (B) Anti-RSV neutralization titers in mouse serum at 4 weeks postinfection analyzed by virus-neutralizing assay. (C) Anti-RSV human IgA antibody levels in BAL fluid analyzed at 7 weeks analyzed by ELISA. In panels A to C, data are shown as the means ± SEMs for 4 to 6 mice per group. (D) Results of analysis of RSV-specific human IFN-γ-positive spleen cells isolated from RSV-infected HIS and NSG mice at 7 weeks by ELISPOT assay.
DISCUSSION
The RSV-infected HIS mice were able to mount innate immune responses, features of RSV respiratory disease, and, importantly, human adaptive immune responses, including RSV-neutralizing antibodies. RSV-neutralizing antibody responses strongly correlate with protection against RSV disease (20, 21) and have not been associated with severe disease following RSV reinfections (22, 23). However, once the RSV infection is established, cellular immunity is required to completely eliminate RSV. Both human humoral neutralizing and human cellular responses were elicited by RSV in the HIS mouse model.
Despite extensive efforts, the realization of a safe and effective RSV vaccine has remained a challenge. HIS mice could be useful not only for the preclinical evaluation of vaccines but also for the identification and development of novel anti-RSV monoclonal antibodies. Although mice are only semipermissive to RSV infection, various murine strains have extensively been used to establish the preclinical features and immunogenicity of RSV vaccine candidates. The further preclinical advancement of vaccines then requires confirmation in other animals as models of human infection, such as cotton rats, cattle, or nonhuman primates. HIS mice could help reduce the number of those studies with larger animals required. In addition, the poor long-term immunity against natural RSV infection and the risk of vaccine-enhanced RSV disease, both of which are major hurdles for the development of RSV vaccines, could be more relevantly assessed with human immune cells. Human CD8 cells require HLA class I molecules in the thymus and other tissues for their development and function (24, 25). Since these HIS mice received only HLA class II and not HLA class I, the human CD8 cells, although present (Table 1), are nonfunctional. Therefore, the RSV-associated lung pathology and immune responses observed are due to the CD4 responses. Given the role of CD4 T cells in RSV disease pathogenesis (26), especially vaccine-enhanced disease (27), and the essential role of neutralizing antibodies in protection against RSV infections (8–10, 21), we focused on the HIS mouse model with functional CD4 T and B cells. It will be interesting to test in future studies the response to RSV infection in the HIS mouse model with CD8 cells to assess the differential roles of CD8 and CD4 cells in RSV-associated pathology. Interestingly, the presence of functional CD4 T and B cells was required to induce mucus hyperplasia, as this response was not induced in the immunodeficient mice after RSV infection.
In addition, there is a need to further define and better understand human RSV lung disease. Only a limited number of human histopathology studies of severe RSV lung disease are available (28–31). The prominent histological features of severe and fatal human RSV disease are peribronchiolar infiltrates of leukocytes, intrabronchiolar plugs with mucus, fibrin and cellular debris of leukocyte and epithelial cells, as well as submucosal edema (28–31). Similar findings were observed in the lungs of RSV-infected HIS mice. An additional feature of human severe RSV disease is secretion of inflammatory cytokines, such as tumor necrosis factor alpha, IL-6, macrophage inflammatory protein 1α/CCL3, and monocyte chemotactic protein 1/CCL2 (32–35). Similar to the findings in RSV-infected HIS mice, the most abundant cells in human BAL fluid are neutrophils (36–38).
The human immune cells in the HIS mice were functional and capable of mounting effective anti-RSV innate and adaptive (both humoral and cellular) immune responses. Therefore, the HIS mice could productively clear the RSV infection, while the infection persisted in the immunodeficient NSG mice. The immune cell-mediated induction of cytokines in response to a pathogen is primarily responsible for the feeling of sickness and the loss of body weight (39, 40). Correspondingly, the robust human cell-derived cytokine response to RSV in HIS mice is likely responsible for the observed loss of body weight. In addition, the cytokines and chemokines recruit immune cells to the site of infection and induce pathology and mucus hyperplasia (41–43), as was observed in RSV-infected HIS mice. The inefficiency with which immunodeficient NSG mice cleared the RSV infection, a lack of weight loss, and the absence of significant changes in lung histopathology are consistent with the findings described in previous reports (44, 45). Similar to the plaque assay, qRT-PCR for the RSV genome showed a lower RSV RNA load in HIS mice than NSG mice (not shown). However, in contrast to the results of the plaque assay, the RNA load is not always a true reflection of infectious RSV titers (46).
In future, it would be interesting to evaluate if the RSV infection of the HIS mice also results in increased airway reactivity, which is often seen in infants with a predisposition for asthma. This could also be studied with models of allergic sensitization. Future studies also need to further define the temporal systemic and lung mucosal cellular responses in this humanized mouse model and to further assess the role of the respiratory epithelial cells in the pathogenesis of RSV infection and protection against RSV disease.
ACKNOWLEDGMENT
The Aaron Diamond AIDS Research Center is an affiliate of The Rockefeller University.
REFERENCES
- 1.Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR, Williams JV, Zhu Y, Grijalva CG, Prill MM, Iwane MK. 2013. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 132:e341–e348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. 2009. The burden of respiratory syncytial virus infection in young children. N Engl J Med 360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 5.Kalina WV, Gershwin LJ. 2004. Progress in defining the role of RSV in allergy and asthma: from clinical observations to animal models. Clin Dev Immunol 11:113–119. doi: 10.1080/10446670410001722131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang K, Varga SM. 2014. Mucosal vaccines against respiratory syncytial virus. Curr Opin Virol 6:78–84. doi: 10.1016/j.coviro.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bem RA, Domachowske JB, Rosenberg HF. 2011. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol 301:L148–L156. doi: 10.1152/ajplung.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. 1981. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 98:708–715. doi: 10.1016/S0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 9.The PREVENT Study Group. 1997. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 99:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 10.The IMpact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531–537. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 11.Billerbeck E, Horwitz JA, Labitt RN, Donovan BM, Vega K, Budell WC, Koo GC, Rice CM, Ploss A. 2013. Characterization of human antiviral adaptive immune responses during hepatotropic virus infection in HLA-transgenic human immune system mice. J Immunol 191:1753–1764. doi: 10.4049/jimmunol.1201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brehm MA, Wiles MV, Greiner DL, Shultz LD. 2014. Generation of improved humanized mouse models for human infectious diseases. J Immunol Methods 410:3–17. doi: 10.1016/j.jim.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich TR, Liu C, Gazumyan A, Schaefer U, Furze RC, Seaman MS, Prinjha R, Tarakhovsky A, Ravetch JV, Nussenzweig MC. 2014. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaiswal S, Smith K, Ramirez A, Woda M, Pazoles P, Shultz LD, Greiner DL, Brehm MA, Mathew A. 2015. Dengue virus infection induces broadly cross-reactive human IgM antibodies that recognize intact virions in humanized BLT-NSG mice. Exp Biol Med (Maywood) 240:67–78. doi: 10.1177/1535370214546273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Li X, Coelho-dos-Reis JG, Wilson JM, Tsuji M. 2014. An AAV vector-mediated gene delivery approach facilitates reconstitution of functional human CD8+ T cells in mice. PLoS One 9:e88205. doi: 10.1371/journal.pone.0088205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Li X, Coelho-Dos-Reis JG, Zhang M, Mitchell R, Nogueira RT, Tsao T, Noe AR, Ayala R, Sahi V, Gutierrez GM, Nussenzweig V, Wilson JM, Nardin EH, Nussenzweig RS, Tsuji M. 2015. Human immune system mice immunized with Plasmodium falciparum circumsporozoite protein induce protective human humoral immunity against malaria. J Immunol Methods 427:42–50. doi: 10.1016/j.jim.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Wendland R, Sung B, Wu W, Grunwald T, Worgall S. 2014. Maternal immunization with chimpanzee adenovirus expressing RSV fusion protein protects against neonatal RSV pulmonary infection. Vaccine 32:5761–5768. doi: 10.1016/j.vaccine.2014.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institutes of Health. 2002. Public Health Service policy on humane care and use of laboratory animals. Office of Laboratory Animal Welfare, National Institutes of Health, Bethesda, MD. [Google Scholar]
- 19.Krause A, Xu Y, Ross S, Wu W, Joh J, Worgall S. 2011. Absence of vaccine-enhanced RSV disease and changes in pulmonary dendritic cells with adenovirus-based RSV vaccine. Virol J 8:375. doi: 10.1186/1743-422X-8-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall CB, Walsh EE, Long CE, Schnabel KC. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 21.Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. 2003. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 21:3479–3482. doi: 10.1016/S0264-410X(03)00355-4. [DOI] [PubMed] [Google Scholar]
- 22.Gimenez HB, Chisholm S, Dornan J, Cash P. 1996. Neutralizing and enhancing activities of human respiratory syncytial virus-specific antibodies. Clin Diagn Lab Immunol 3:280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham BS. 2011. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev 239:149–166. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bushkin Y, Demaria S, Mohagheghpour N, Le JM. 1990. Activation of human CD8-positive T cells via the CD8/HLA class I complex. Cell Immunol 126:185–195. doi: 10.1016/0008-8749(90)90311-E. [DOI] [PubMed] [Google Scholar]
- 25.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. 2009. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol 9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 26.Christiaansen AF, Knudson CJ, Weiss KA, Varga SM. 2014. The CD4 T cell response to respiratory syncytial virus infection. Immunol Res 59:109–117. doi: 10.1007/s12026-014-8540-1. [DOI] [PubMed] [Google Scholar]
- 27.Castilow EM, Olson MR, Varga SM. 2007. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res 39:225–239. doi: 10.1007/s12026-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 28.Aherne W, Bird T, Court SD, Gardner PS, McQuillin J. 1970. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol 23:7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. 2007. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 30.Reed JL, Brewah YA, Delaney T, Welliver T, Burwell T, Benjamin E, Kuta E, Kozhich A, McKinney L, Suzich J, Kiener PA, Avendano L, Velozo L, Humbles A, Welliver RC Sr, Coyle AJ. 2008. Macrophage impairment underlies airway occlusion in primary respiratory syncytial virus bronchiolitis. J Infect Dis 198:1783–1793. doi: 10.1086/593173. [DOI] [PubMed] [Google Scholar]
- 31.Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, McKinney L, Reed JL, Welliver RC Sr. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis 195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeVincenzo JP, Wilkinson T, Vaishnaw A, Cehelsky J, Meyers R, Nochur S, Harrison L, Meeking P, Mann A, Moane E, Oxford J, Pareek R, Moore R, Walsh E, Studholme R, Dorsett P, Alvarez R, Lambkin-Williams R. 2010. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 182:1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison AM, Bonville CA, Rosenberg HF, Domachowske JB. 1999. Respiratory syncytical virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am J Respir Crit Care Med 159:1918–1924. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- 34.McNamara PS, Flanagan BF, Hart CA, Smyth RL. 2005. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis 191:1225–1232. doi: 10.1086/428855. [DOI] [PubMed] [Google Scholar]
- 35.Melendi GA, Laham FR, Monsalvo AC, Casellas JM, Israele V, Polack NR, Kleeberger SR, Polack FP. 2007. Cytokine profiles in the respiratory tract during primary infection with human metapneumovirus, respiratory syncytial virus, or influenza virus in infants. Pediatrics 120:e410–e415. doi: 10.1542/peds.2006-3283. [DOI] [PubMed] [Google Scholar]
- 36.Bem RA, Bos AP, Bots M, Wolbink AM, van Ham SM, Medema JP, Lutter R, van Woensel JB. 2008. Activation of the granzyme pathway in children with severe respiratory syncytial virus infection. Pediatr Res 63:650–655. doi: 10.1203/PDR.0b013e31816fdc32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, Sewell HF, Milner AD. 1994. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child 71:428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNamara PS, Ritson P, Selby A, Hart CA, Smyth RL. 2003. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child 88:922–926. doi: 10.1136/adc.88.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burfeind KG, Michaelis KA, Marks DL. 3 November 2015. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin Cell Dev Biol. doi: 10.1016/j.semcdb.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthys P, Billiau A. 1997. Cytokines and cachexia. Nutrition 13:763–770. doi: 10.1016/S0899-9007(97)00185-8. [DOI] [PubMed] [Google Scholar]
- 41.Lee YT, Ko EJ, Hwang HS, Lee JS, Kim KH, Kwon YM, Kang SM. 2015. Respiratory syncytial virus-like nanoparticle vaccination induces long-term protection without pulmonary disease by modulating cytokines and T-cells partially through alveolar macrophages. Int J Nanomedicine 10:4491–4505. doi: 10.2147/IJN.S83493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller AL, Strieter RM, Gruber AD, Ho SB, Lukacs NW. 2003. CXCR2 regulates respiratory syncytial virus-induced airway hyperreactivity and mucus overproduction. J Immunol 170:3348–3356. doi: 10.4049/jimmunol.170.6.3348. [DOI] [PubMed] [Google Scholar]
- 43.Meager A, Wadhwa M. 19 September 2013. An overview of cytokine regulation of inflammation and immunity. eLS. doi: 10.1002/9780470015902.a0024658. [DOI] [Google Scholar]
- 44.Graham BS, Bunton LA, Wright PF, Karzon DT. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest 88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins PL, Graham BS. 2008. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, Guarino B, Silacci C, Marcandalli J, Marsland BJ, Piralla A, Percivalle E, Sallusto F, Baldanti F, Lanzavecchia A. 2013. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature 501:439–443. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]