FIG 4.
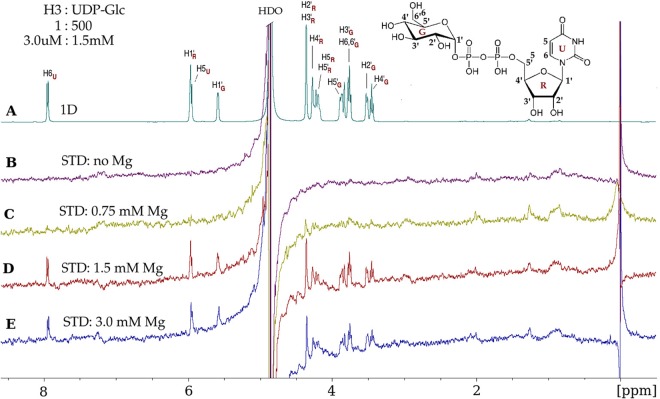
Vaccinia virus H3 protein binds UDP-glucose and requires Mg2+. (A) 1D 1H NMR spectrum of UDP-glucose (UDP-Glc) in the presence of the H3 protein, at a protein-to-ligand molar ratio of 1:500 (3.0 μM–1.5 mM). UDP-Glc protons, with glucose (G), ribose (R), and uracil (U) subscripts, are identified on the spectrum and on the UDP-Glc chemical structure (inset). (B) No STD effects are observed in the absence of Mg2+. (C) A few STD signals were observed at 0.75 mM Mg2+. (D) Maximal STD signals from UDP-Glc protons were observed at 1.5 mM Mg2+, which are the result of UDP-Glc molecules gaining magnetic saturation while bound to H3. After dissociating from H3, the UDP-Glc molecules retain saturation and contribute to the STD spectrum while unbound (free). (E) At 3 mM Mg2+, there is no further enhancement of the STD signals. The STD data were acquired with a spectral width of 6,000 Hz, 32,768 data points, 2,048 scans, and a receiver gain of 128 and without water suppression. The saturation of the protein was achieved by selective irradiation at −0.15 ppm (see Materials and Methods). “HDO,” residual partially deuterated water resonance.