FIG 5.
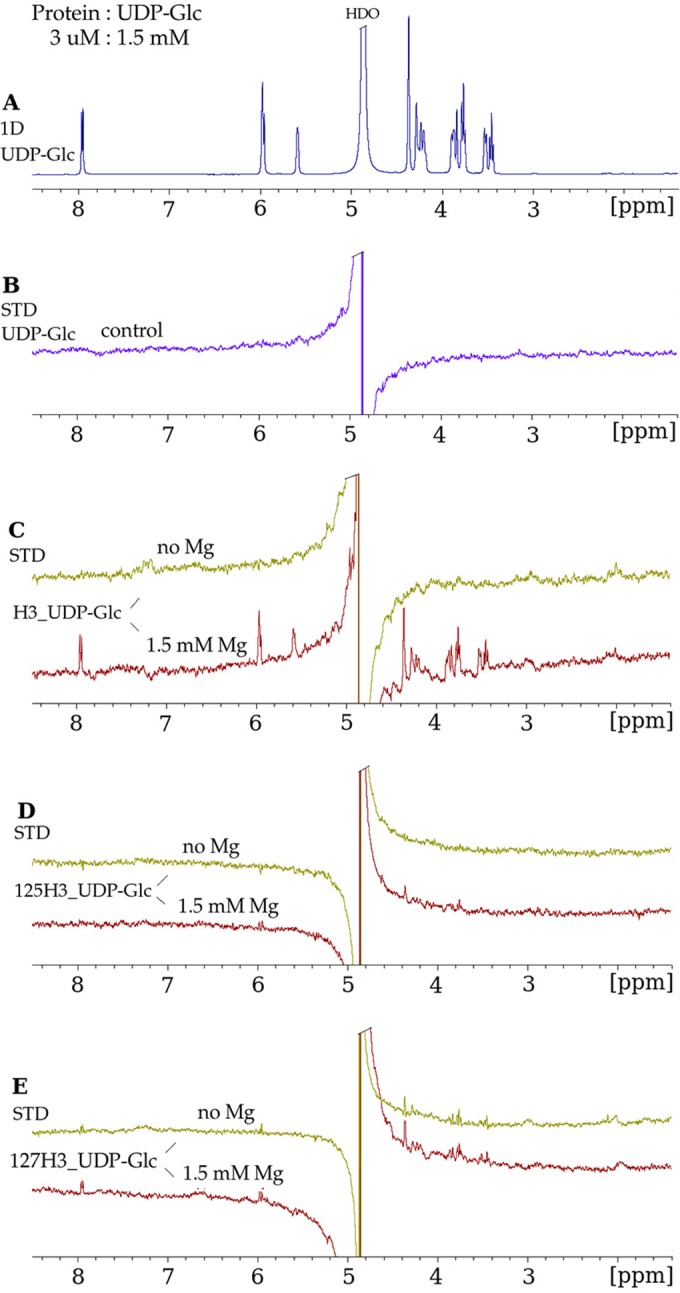
Mutation of the conserved glycosyltransferase D/ExD motif in H3 disrupts UDP-Glc binding. (A) 1D 1H NMR spectrum of UDP-Glc in the presence of H3, at a protein-to-ligand molar ratio of 1:500, under the same conditions as those described in the legend of Fig. 4A. (B) STD NMR spectrum of UDP-Glc in the absence of the H3 protein, in which no STD enhancements were observed for the protons of UDP-Glc. (C) STD NMR spectra in the absence of Mg2+ and in the presence of Mg2+ demonstrating binding of UDP-Glc to wild-type H3. (D) E125A mutant (“125H3_UDP-Glc”). The strong decrease in the STD enhancements for UDP-Glc in the presence of the E125A mutant indicates that the mutation of the H3 protein at residue 125 inhibits the binding of UDP-Glc. (E) D127A mutant (“127H3_UDP-Glc”). The lack of Mg2+ dependence of the STD enhancements in the presence of the D127A mutant suggests nonspecific binding.
