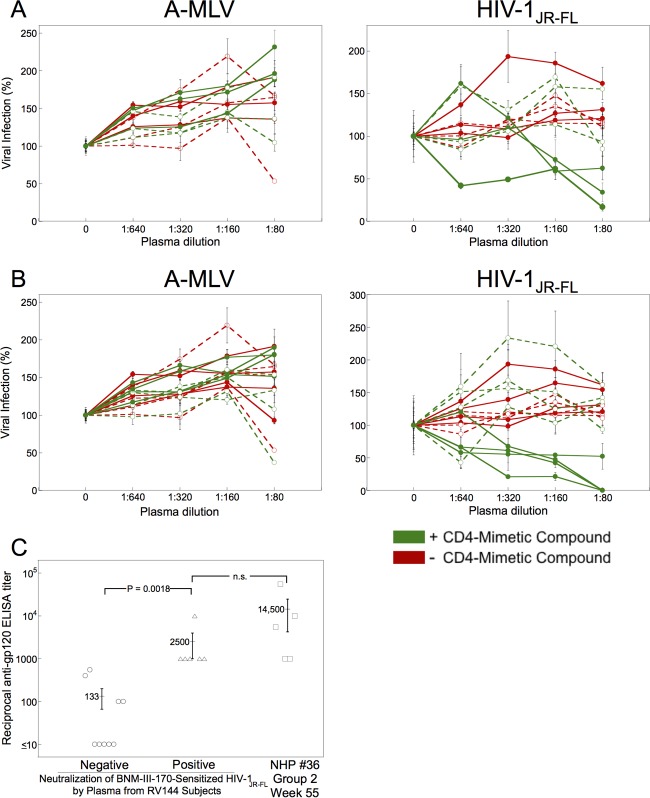
FIG 5.
Some RV144 vaccinees generate antibodies that neutralize sensitized HIV-1. (A) Recombinant, luciferase-expressing viruses with HIV-1JR-FL Env or A-MLV Env were incubated with 30 μM BNM-III-170 (green) or without compound (red). The viruses subsequently were incubated with the indicated dilution of plasma from human subjects in the RV144 HIV-1 vaccine trial. Preimmune plasma samples are designated by open symbols and dashed lines and immune plasma samples by closed symbols and unbroken lines. The virus-plasma mixtures were incubated with Cf2Th-CD4/CCR5 cells for 48 h, after which the cells were lysed and luciferase activity measured. The level of infection relative to that seen in the absence of plasma is shown. The means and standard deviations from triplicate samples within an experiment are shown; the experiment was performed twice with similar results. (B) Neutralization assays were performed as described for panel A, except that 30 μM BNM-IV-147 was used instead of BNM-III-170. (C) The reciprocal titers of anti-gp120 antibodies measured by ELISA are shown for immune plasma from RV144 vaccinees that are either negative or positive for the neutralization of BNM-III-170-sensitized HIV-1JR-FL. The anti-gp120 antibody reciprocal titers of plasma from monkeys in group 2 of the NHP 36 study are shown for comparison. Differences between the groups were analyzed by the Mann-Whitney U (t) test; the P value is shown for the comparison where statistical significance was achieved. n.s., not significant.