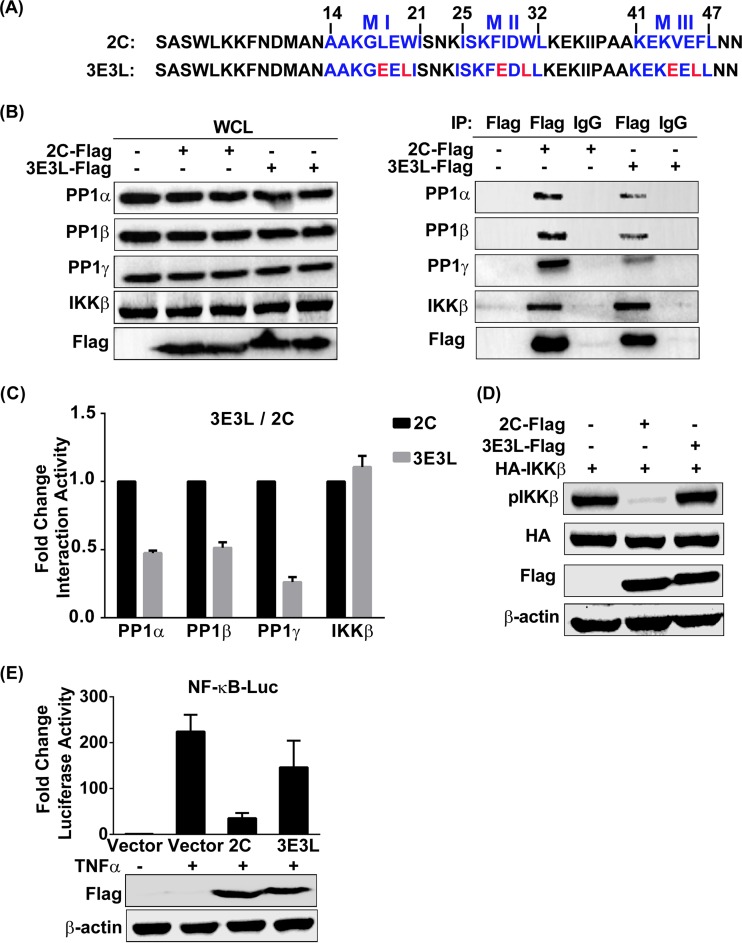
FIG 5.
A PP1-binding-deficient mutant of EV71 2C barely inhibits IKKβ phosphorylation and NF-κB activation. (A) Analysis of the protein sequences of wild-type 2C in comparison with those of 2C mutant 3E3L. (B) 293T cells were transfected with the vector, 2C-Flag, or the Flag-tagged 3E3L mutant. At 24 h posttransfection, cells were harvested and lysed. The whole-cell lysate was immunoprecipitated with anti-Flag or control mouse IgG, and the immunoblots were probed with the corresponding antibodies. (Left) Confirmation of expression of the indicated protein by immunoblotting; (right) detection of endogenous PP1 in an immunoprecipitated sample. (C) Quantification of the proteins in the IP panels from panel B by using ImageJ software. The results shown (mean ± SD fold change) are representative of those from three independent experiments. (D) 293T cells were cotransfected with HA-IKKβ and the vector, 2C-Flag, or 3E3L mutant-Flag at 24 h posttransfection, and the whole-cell lysate was immunoblotted by using the indicated antibodies, including a phospho-IKKα/β (Ser-176/180)-specific antibody. (E) 293T cells were transfected with plasmids carrying the indicated protein or the vector alone and then mock treated or treated for 6 h with TNF-α (10 ng/ml) at 24 h posttransfection. Cells were lysed and analyzed by a luciferase reporter assay. The data shown (mean ± SD fold change) are representative of those from three independent experiments.