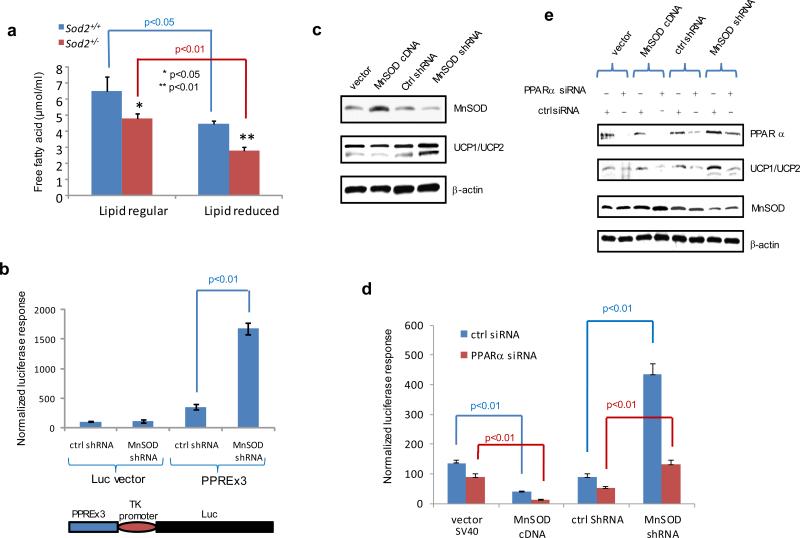
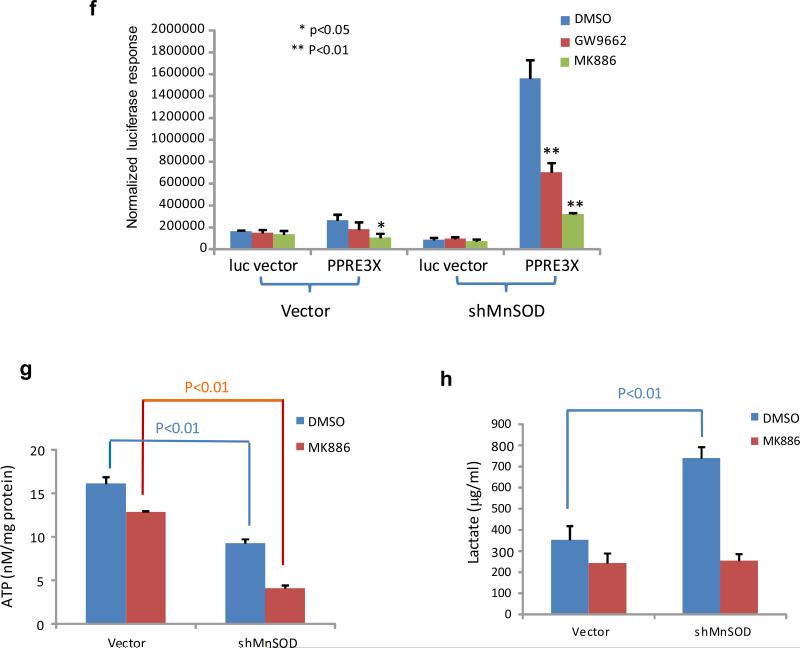
Figure 4.
Free fatty acid-activated PPARα-mediated transcription of the UCP1 gene under MnSOD-deficient conditions. (a) The Sod2+/− and Sod2+/+ primary cells were cultured in DMEM and lipid-reduced DMEM media, and the concentrations of free fatty acids in the cells were measured. (b) PPAR elements located in the UCP1 promoter region were linked to the TK basal promoter to drive the luciferase reporter gene (bottom). The reporter construct was transiently transfected into mouse skin JB6 cells, and induction of the reporter gene was carried out in the presence or absence of MnSOD silencing. Reporter activity was normalized to cotransfected β-galactosidase activity. The empty pGL3 vector was also transfected as a control. (c) The JB6 cells were stably transfected with MnSOD cDNA or injected with MnSOD lentiviral shRNA. The levels of MnSOD and related UCP1/UCP2 proteins were measured. (d) The luciferase reporter construct was cotransfected with PPARα siRNA into the JB6 cells with different levels of MnSOD. A vector containing a SV40 promoter alone was used as a control. The luciferase reporter responses were analyzed. (e) The corresponding proteins in transfected JB6 cells were measured to confirm the reporter responses. (f) The reporter construct was transfected into JB6 cells, and then treated with PPARα inhibitor MK886 or PPARγ inhibitor GW9662; the normalized reporter responses were estimated. (g,h) After MnSOD-silenced JB6 cells (shMnSOD) were treated with MK886, the productions of ATP and lactate were measured.