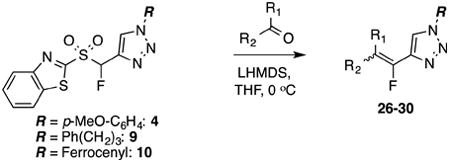
Table 3. Condensations of sulfones 4, 9, and 10 with ketones.
| ||||
|---|---|---|---|---|
|
| ||||
| entry | sulfone | R1R2CO | product, yield,a % isomer ratiob | 19F NMR δ (ppm)c |
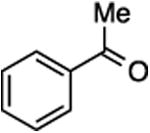
| 1 | 4 |
|
26, 87%, NAd | –121.99 ppmd |
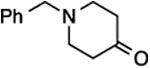
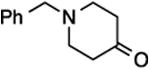
| 2 | 9 |
|
27, 58%, NA | –121.52 ppm |
| 3 | 4 |
|
28, 64%,e 28:72 | –116.46 ppm (minor)f –116.57 ppm (major)f |
| 4 | 9 |
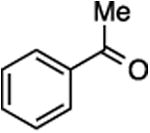
|
29, 77%,e 20:80 | –115.56 ppm (minor E-29)g –116.33 ppm (major Z-29)g |
| 5 | 10 |
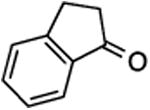
|
30, 58%, one isomer onlyh | –123.76 ppm |
Yields are of isolated and purified products.
Olefin isomer ratios in the crude reaction mixtures were determined by 19F NMR prior to isolation.
Referenced to CFCl3 as internal standard; 282 MHz, CDCl3 solvent.
Data reported in ref 11a.
Combined yield of E and Z-isomers.
Stereochemistry of the isomers was assigned by comparison to compound 29 (see text).
Stereochemistry of the major isomer was assigned by X-ray crystallography (see text).
Stereochemistry was not assigned.
