Figure 1. Full-length sgRNA drives inward lobe closure of Cas9.
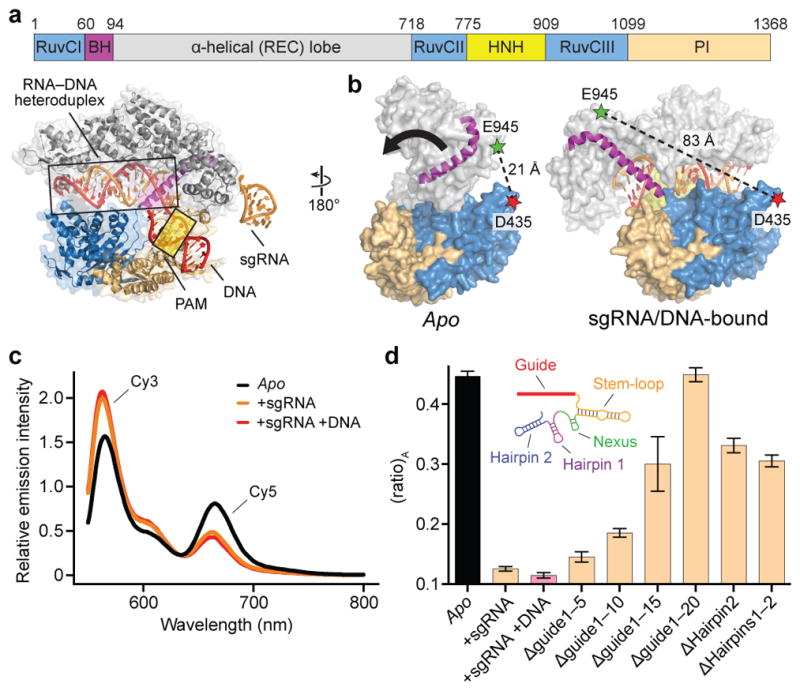
a, Domain organization of S. pyogenes Cas9 (top) and X-ray crystal structure of sgRNA/DNA-bound Cas9 (PDB ID 4UN3, ref. 16) (bottom), with HNH domain omitted for clarity. BH, bridge helix; REC, recognition; PI, PAM-interacting. b, Design of Cas9hinge FRET construct. Measured distances between D435 and E945 in apo (PDB ID 4CMP, ref. 13) and sgRNA/DNA-bound Cas9 structures are indicated. Inward lobe closure is exemplified by movement of the BH (arrow). Regions of the PI domain, sgRNA, and DNA are omitted for clarity. c, Fluorescence emission spectra for Cas9hinge in the presence of the indicated substrates. d, (ratio)A data for Cas9hinge. Inset: schematic of full-length sgRNA coloured by motif29. Error bars represent the standard deviation; n=3.
