Fig. 1.
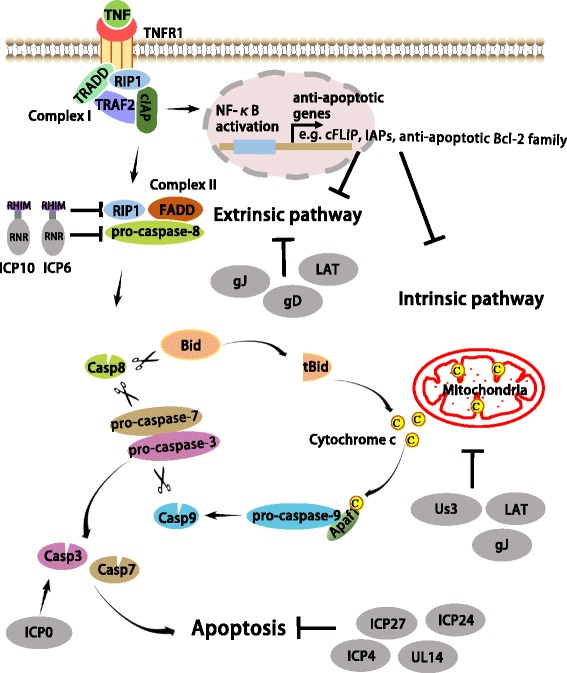
HSV modulates apoptosis signaling. Apoptosis is activated through both extrinsic and intrinsic pathways. In the extrinsic pathway, the ligation of a death receptor (e.g. tumor necrosis factor receptor-1; TNFR1) by its ligand promotes the assembly of the membrane-associated Complex I, which is composed of TRADD, TRAF2, RIP1, and cIAPs. This complex can activate NF-κB that initiates the transcription of some anti-apoptosis genes, such as cFLIP, IAPs and anti-apoptotic Bcl-2 family. During apoptosis, Complex I eventually triggers formation of Complex II, which is composed of FADD, RIP1, and pro-caspase-8, and leads to the activation of caspase-8. In the intrinsic pathway, cytochrome c is released from mitochondria to the cytoplasm, causing the formation of the apoptosome with Apaf-1 and pro-caspase-9, resulting in the activation of caspase-9. Activated caspase-8 and caspase-9 in turn cleave and activate downstream executioner caspases such as caspase-3 and caspase-7 for the execution of apoptosis. Activated caspase-8 also can engage the intrinsic pathway via cleavage of Bid to form tBid. HSV encodes anti-apoptotic viral proteins to subvert apoptotic signaling. Us3 can block the intrinsic pathway while R1 and LAT prevent the extrinsic pathway. gJ and LAT blocks both the intrinsic and the extrinsic pathways. gD activates NF-κB to enhancing the expression of anti-apoptotic genes. The regulatory proteins ICP4, ICP24 and ICP27 may indirectly inhibit apoptosis by promoting the production of later anti-apoptotic viral gene products. UL14 has HSP-like functions that may block caspases activation and apoptosis. Conversely, ICP0 promotes caspase activation and apoptosis
