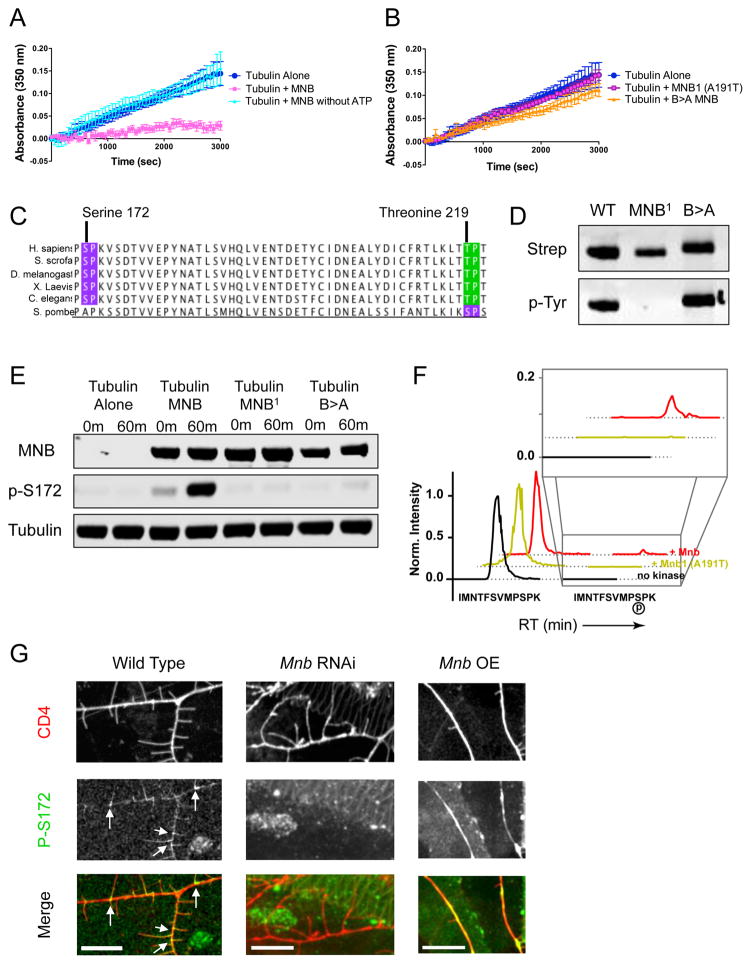
Figure 5. Mnb inhibits microtubule polymerization in vitro by phosphorylating β-tubulin at serine-172.
(A) Tubulin polymerization determined via turbidity (at 350nm) in the absence of MNB (dark blue), in the presence of MNB (pink), and in the presence of MNB without ATP (light blue). (B) Tubulin polymerization determined via turbidity in the presence of either the MNB1 (A191T) mutant MNB (pink/purple) or the B>A mutant MNB (orange) (P = 0.0007 and 0.004 for MNB1 and B>A MNB vs. WT MNB). All turbidity assays were conducted at 25ºC in BRB80 buffer with 25 μM tubulin, 5 μM GFP-MNB, 1 mM GTP and 1 mM ATP, unless otherwise noted. Means ± s.d. are plotted from at least n = 3 experiments per condition. (C) Sequence analysis of β-tubulin shows two potential MNB phosphorylation sites: serine 172 (purple) and threonine 219 (green), both of which are highly conserved. (D) Immunoblots of total MNB protein and autophosphorylated MNB protein detected by antibodies against the strep-tag and phosphotyrosine, respectively, show that wild type and B>A MNB recombinant proteins are active kinases, while MNB1 is not. (E) Immunoblots of in vitro kinase assays with samples taken at 0 min and 60 min after incubation of 500 nM tubulin and 1 mM ATP alone, or with 500 nM wild type, MNB1 or B>A MNB at 25ºC. MNB proteins were detected by an anti-strep antibody, β-tubulin phosphorylated at serine-172 was detected by a phospho-specific antibody, and total tubulin was detected by an anti-alpha-tubulin antibody. (F) Extracted ion chromatogram from MS1 filtering of LC-MS/MS data shows the unmodified peptide, IMNTFSVMPSPK, containing Ser172 (earlier retention time peak) and the phosphorylated Ser172 peptide (later retention time and inset). The MS1 intensity is normalized to the peak of the unmodified peptide identified in each sample. The phosphorylated Ser172 peptide appears in the presence of wild type MNB protein, but does not appear in the presence of the kinase dead mutant, MNB1, or in the absence of the kinase. See also Dataset S1. (G) Immunohistochemical staining of wild type, mnb RNAi and mnb OE larval fillets with anti-phospho-S172 and anti-GFP (against the membrane marker, CD4). Phospho-S172 is normally present at the base of terminal branches in wild type class III neurons (white arrows), but expression decreases in mnb RNAi neurons and increases in mnb overexpressing neurons. Scale bars are 10 μm.