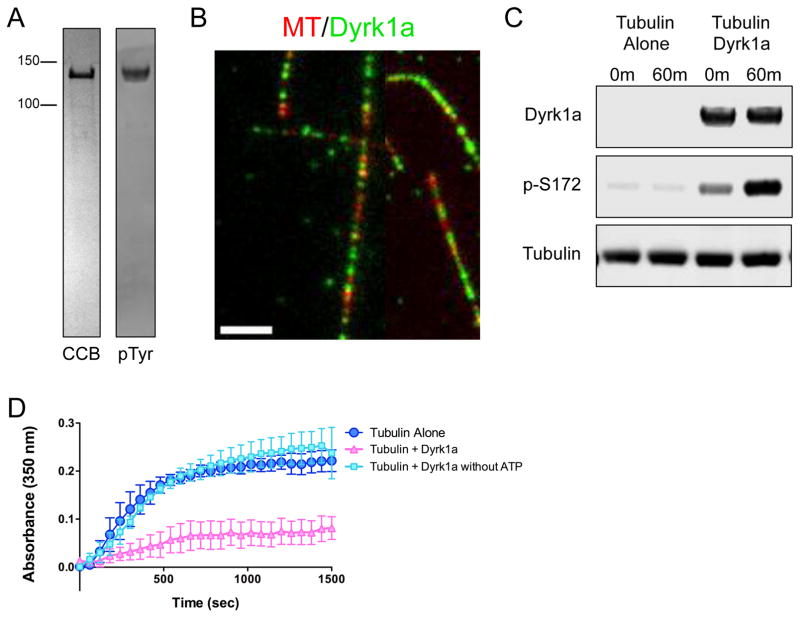
Figure 6. Mammalian DYRK1a exhibits a conserved mechanism of action on tubulin.
(A) CBB stained SDS-PAGE of purified recombinant GFP-tagged DYRK1a kinase (left), and an immunoblot of DYRK1a protein detected by an antibody against phosphotyrosine (right) to show that the recombinant GFP-DYRK1a kinase is active and able to autophosphorylate its own tyrosine residues. (B) TIRF-M reveals GFP-DYRK1a (green) binds along the lattice of taxol-stabilized MTs (red) in vitro (scale bar is 2.5 μm). (C) Immunoblots of in vitro kinase assays with samples taken at 0 min and 60 min after incubation of 500 nM tubulin and 1 mM ATP alone, or with 500 nM GFP-DYRK1a at 25ºC. DYRK1a protein was detected by an anti-strep antibody, β-tubulin phosphorylated at serine-172 was detected by a phospho-specific antibody, and total tubulin was detected by an anti-alpha-tubulin antibody. (D) Tubulin polymerization determined via turbidity (at 350nm) in the absence of DYRK1a (dark blue), in the presence of DYRK1a (pink), and in the presence of DYRK1a without ATP (light blue). All turbidity assays were conducted at 37ºC in BRB80 buffer with 25 μM tubulin, 5 μM GFP-DYRK1a, 1 mM GTP and 1 mM ATP, unless otherwise noted. Means ± s.d. are plotted from at least n = 3 experiments per condition.