Fig. 1.
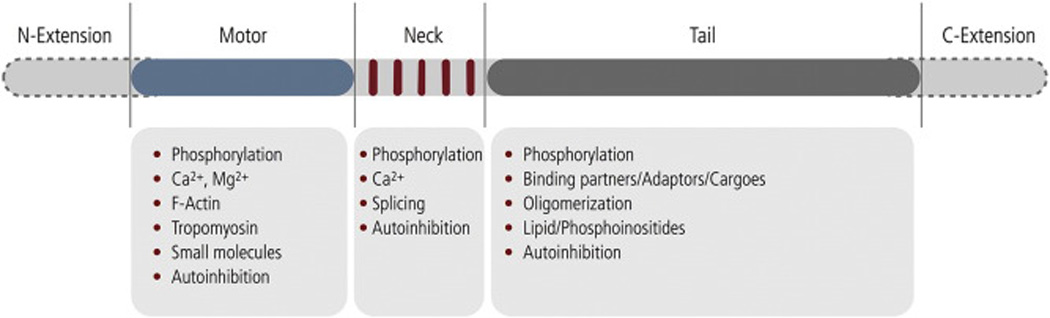
Basic building blocks of the myosin heavy chain: A myosin heavy chain consists of a prototypic motor domain (blue) which comprises the ATP binding site and an actin binding region, a light chain binding neck region containing multiple IQ-motifs (red), and a tail region (grey). N- and C-terminal extensions (grey dashed line) for the heavy chain are reported that may include N-terminal kinase domains, ATP-insensitive actin binding sites. Myosin light chains noncovalently associate with the myosin heavy chain to form the myosin holoenzyme. Moreover, myosin light chains stabilize and prime the neck region to competently function as a rigid lever that rotates relative to the myosin motor domain during the force generating power stroke that translocates myosin along actin [5]. Allosteric feedback mechanisms discussed in the present review target the motor domain, the neck domain and the tail region of the heavy chain and are listed below the respective domain. The N-terminal fraction of the molecule including the neck region and the associated light chains is referred to as S1. A longer fragment which additionally includes a portion of the tail domain is referred to as HMM. S1 fragments are monomeric whereas HMM fragments are dimeric. S1 is inherently active and serves as a powerful surrogate to study the transient-kinetic properties of the isolated myosin motor domain.
