Abstract
Electrical stimulation of the pudendal nerve (PN) is a promising approach to restore continence and micturition following bladder dysfunction resulting from neurological disease or injury. Although the pudendo-vesical reflex and its physiological properties are well established, there is limited understanding of the specific neural mechanisms that mediate this reflex. We sought to develop a computational model of the spinal neural network that governs the reflex bladder response to PN stimulation. We implemented and validated a neural network architecture based on previous neuroanatomical and electrophysiological studies. Using synaptically-connected integrate and fire model neurons, we created a network model with realistic spiking behavior. The model produced expected sacral parasympathetic nucleus (SPN) neuron firing rates from prescribed neural inputs and predicted bladder activation and inhibition with different frequencies of pudendal afferent stimulation. In addition, the model matched experimental results from previous studies of temporal patterns of pudendal afferent stimulation and selective pharmacological blockade of inhibitory neurons. The frequency- and pattern-dependent effects of pudendal afferent stimulation were determined by changes in firing rate of spinal interneurons, suggesting that neural network interactions at the lumbosacral level can mediate the bladder response to different frequencies or temporal patterns of pudendal afferent stimulation. Further, the anatomical structure of excitatory and inhibitory interneurons in the network model was necessary and sufficient to reproduce the critical features of the pudendo-vesical reflex, and this model may prove useful to guide development of novel, more effective electrical stimulation techniques for bladder control.
Keywords: Electrical stimulation, Neural network, Computational model, Integrate and fire neuron, Pudendal nerve stimulation
1 Introduction
Bladder dysfunction resulting from neurological disease or injury, such as spinal cord injury (SCI), produces symptoms of urinary incontinence, chronic retention of urine, and detrusor sphincter dyssynergia (Abrams et al. 2002), which greatly reduce quality of life (Anderson 2004; Ku 2006). Peripheral nerves that innervate the lower urinary tract (e.g., pelvic and pudendal nerves) provide motor control and sensory feedback that is critical for normal urinary function (de Groat et al. 2015). Electrical stimulation of pudendal afferents is a promising method to restore continence and micturition through reflex inhibition or excitation of the bladder by the activation of spinal circuits (Boggs et al. 2006; Woock et al. 2008). Although the input–output properties of the pudendo-vesical reflex have been characterized empirically, there is limited understanding of the underlying neural network mechanisms that mediate the reflexes governing the effects of PN stimulation on bladder function. The objective of this work was to develop and validate a biophysically-motivated model of the neural network underlying the pudendo-vesical reflex.
Several key features of the bladder response to pudendal afferent stimulation indicate that the pudendo-vesical reflex is a spinal network-mediated phenomenon, rather than a product of higher order processing in the brainstem. Electrical stimulation of pudendal afferents generates robust bladder contractions in animals (Tai et al. 2006; Yoo and Grill 2007; Yoo et al. 2008) and humans (Yoo et al. 2007; Yoo et al. 2011), but the excitatory pudendo-vesical reflex is strongly dependent on bladder volume (Woock et al. 2011), suggesting a convergence of pelvic and pudendal afferents in the spinal cord network (Woock et al. 2011). The effects of PN afferent stimulation on the bladder are strongly dependent on the frequency of stimulation, with high frequencies evoking reflex bladder contractions and low frequencies producing bladder inhibition (Boggs et al. 2006; Woock et al. 2008; Yoo et al. 2008). Although there are multiple reflex pathways that may be activated by PN stimulation (e.g., cranial sensory branch stimulation produces bladder activation at low frequencies via a supraspinal mechanism (Yoo et al. 2008)), the frequency-dependent effects of dorsal genital branch of the PN pathway are mediated in lumbosacral spinal neural networks, as they are preserved after spinal cord transection (Woock et al. 2008). Finally, eliminating pudendal sensory feedback by nerve transection in animal experiments (Peng et al. 2008) and intraurethral anesthesia in humans (Shafik et al. 2003) reduces voiding efficiency, indicating that these feedback signals critically interact with the network that produces normal voiding behavior. Collectively, these studies indicate that interactions between neurons in a lumbosacral spinal network are responsible for the pudendo-vesical reflex.
A limited number of quantitative models have been developed that explore the spinal neural network control of bladder function. Of the models that describe control of the lower urinary tract, most do not model neural activity with sufficient detail or include contributions by pudendal afferents. Hosein and Griffiths (1990) developed a quantitative computer simulation of a proposed lower urinary tract control system, simulating changes in bladder volume, pressure, and flow rate from inhibitory and excitatory control signals that represented neural activity in the brainstem and peripheral nerves. Bastiaanssen et al. (1996) developed a biomechanical neuromuscular model of the bladder that incorporated neuroanatomy, physiology, and muscular mechanisms, and responded to input signals from a simulated neural network. A computer model of the neural control and mechanical properties of the bladder and urethra by van Duin et al. (1999) demonstrated the importance of contributions by urethral afferents for simulation of normal lower urinary tract behavior. A more recent model of the periaqueductal gray (PAG) and pontine micturition center (PMC) in the brainstem reproduced the micturition cycle, but did not include pudendal afferents or the associated spinal reflex pathways (de Groat and Wickens 2013).
The goal of this work was to develop a computational model of the lumbosacral spinal neural network responsible for the dorsal genital pathway of the pudendo-vesical reflex. The model structure was based upon established neuroanatomical connections and inspired by a prior model of frequency-dependent selection of spinal locomotor reflexes (Jilge et al. 2004), as the lower urinary tract reflexes evoked by pudendal afferent stimulation similarly depend on the frequency of afferent activation (Boggs et al. 2006). Our model of the neural network that mediates the spinal pudendo-vesical reflex replicated the effects of pudendal afferent stimulation frequency and pattern on bladder pressure measured experimentally and enabled dissection of the underlying mechanisms.
2 Methods
We developed, implemented, and validated a computational model of the spinal neural network mediating the dorsal genital pathway of the pudendo-vesical reflex.
2.1 Model topology
The network model incorporated sensory inputs from pelvic and pudendal nerve afferent fibers, excitatory and inhibitory interneurons, and preganglionic pelvic efferent neurons in the sacral parasympathetic nucleus (SPN) that innervate the bladder via the pelvic nerve (Fig. 1). A simplified descending excitatory pathway from the PAG and PMC was included to generate distension-evoked contractions (DECs). Table 1 includes a description of each neuron and the references that informed the structure of the model.
Fig. 1.
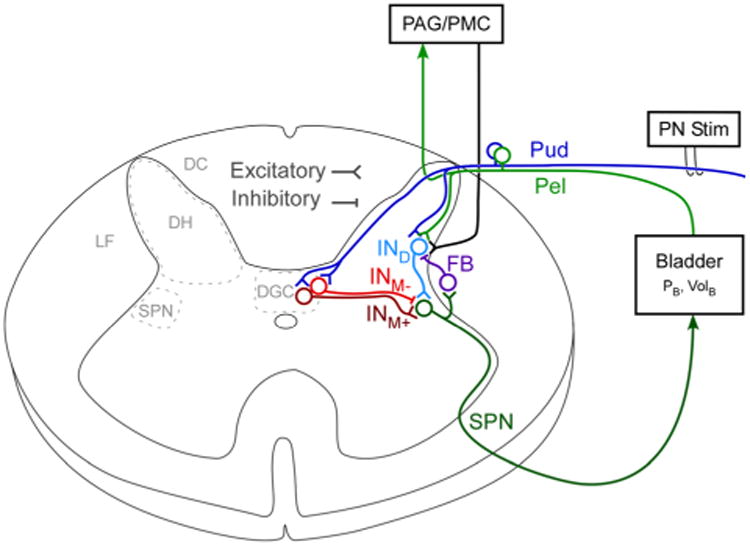
Structure of neural network model of the pudendo-vesical reflex. The network was composed of linear integrate and fire (LIF) neurons. Pudendal afferents arising from the dorsal genital pathway innervate both medial and lateral portions of the dorsal horn (DH) and were represented by a single neuron with projections onto dorsal and medial interneurons (Pud). Pelvic afferents make primarily lateral projections in the dorsal horn and were represented by a single neuron (Pel). The dorsal interneuron (IND) received input from both pelvic and pudendal nerves, while two interneurons (INM+, INM-) in the dorsal gray commissure (DGC) provided excitatory and inhibitory control of the sacral parasympathetic nucleus (SPN) neuron, the neural output to the bladder. The feedback interneuron (FB) provided negative feedback from the SPN on the dorsal interneuron. Pelvic afferents also projected via the dorsal columns (DC) to a supraspinal processing node that simulated the periaqueductal gray (PAG) and pontine micturition center (PMC) and provided descending excitation of the IND via the lateral funiculus (LF)
Table 1. Anatomical and electrophysiological references for model topology.
| Neuron | Description | Neuroanatomy | Animal model & references |
|---|---|---|---|
| Pud | Pudendal afferents | Pudendal afferents enter the dorsal horn of the sacral spinal cord. Medial projections innervate the dorsal gray commissure (DGC) and lateral projections innervate the lateral edge of the dorsal horn. | rhesus monkey, cat (Roppolo et al. 1985; Thor et al. 1989) |
| Pel | Pelvic afferents | Pelvic afferents enter the dorsal horn of the sacral spinal cord. Pelvic afferents primarily make lateral projections along the lateral border of the dorsal horn. | rhesus monkey, rat (Nadelhaft et al. 1983; Roppolo et al. 1985; Sengupta and Gebhart 1994) |
| INM+ | Excitatory interneuron | Interneuron that excites neurons in the sacral parasympathetic nucleus (SPN), located medial to the SPN. | cat, rat (de Groat et al. 1998) |
| INM- | Inhibitory interneuron | Interneuron that inhibits neurons in the SPN, located medial to SPN. GABAergic neurons project from DGC and dendrites from SPN have GABAergic receptors. | rhesus monkey, cat, rat (Nadelhaft et al. 1983; Nadelhaft and Booth 1984; de Groat et al. 1998) |
| IND | Excitatory interneuron | Interneuron along lateral edge of dorsal horn that receives input from peripheral nerve afferents and excites the SPN. | cat, rat (Araki and de Groat 1997; de Groat et al. 1998) |
| FB | Inhibitory feedback interneuron | Feedback interneuron that is controlled by output of SPN. This interneuron inhibits the dorsal interneuron and incoming peripheral afferents. | cat, rat (de Groat and Ryall 1968; de Groat 1976; Shefchyk 2001) |
| PMC | Pontine Micturition Center | Descending pathway from the PMC produces polysynaptic excitation of the SPN. | cat (Sasaki and Sato 2013) |
| SPN | SPN neuron (Pelvic efferent) | Neurons with cell bodies in the SPN make up the efferent pelvic nerve. | cat (de Groat et al. 1982; Sasaki 1998) |
Pudendal afferents have cell bodies in the dorsal root ganglia and enter the dorsal horn at the sacral level (L6-S1 in rats, S1-S3 in cats, and S2-S4 in humans), diverging into both medial and lateral projections (Roppolo et al. 1985; Thor et al. 1989). The lateral projections synapse on interneurons along the lateral edge of the dorsal horn, while the medial projections synapse on interneurons in the dorsal gray commissure (DGC) or send rostral projections via the dorsal columns. In this model, we excluded ascending projections by pudendal afferents and focused on the local spinal reflex-mediated mechanisms of bladder control with electrical stimulation.
Pelvic afferents, also with cell bodies in the dorsal root ganglia, enter the dorsal horn at the sacral level (L6-S1 in rats, S1-S3 in cats, and S2-S4 in humans). Pelvic afferents make predominately lateral projections (Nadelhaft et al. 1983; Roppolo et al. 1985) to the dorsal interneurons, in addition to rostral projections to the brainstem. Pelvic mechanoreceptors in the bladder are sensitive to changes in bladder pressure, increases in tension of the bladder wall, or bladder distension. The pelvic afferent neuron in this network was modeled to match the response of low threshold mechanoreceptors sensitive to bladder distension and was based upon a selected individual response to bladder distension from examples of low threshold type neurons in Sengupta and Gebhart (1994). Bladder activation is via the output of the pelvic preganglionic efferent neurons with cell bodies in the SPN (de Groat et al. 1982).
Both excitatory and inhibitory medial interneurons modulate SPN output (pelvic efferents) through synaptic connections onto neurons in the SPN (de Groat et al. 1998). Additionally SPN neurons have dendrites that extend to the DGC (Nadelhaft et al. 1983; Nadelhaft and Booth 1984), where medial interneurons are likely to originate. Stimulation of the DGC evokes coordinated increases in bladder pressure and decreases in urethral sphincter pressure, further suggesting it is a critical part of the network controlling micturition (Blok et al. 1998; Grill et al. 1999; Pikov et al. 2007). The dorsal interneuron receives inputs from pelvic and pudendal afferents and acts as an excitatory input to the SPN (Araki and de Groat 1997). The inhibitory feedback interneuron (FB) is excited by the SPN neuron, and provides negative feedback to modulate the transmission of incoming afferent activity (de Groat and Ryall 1968; de Groat 1976; Shefchyk 2001).
A supraspinal node, which simulated sensory integration performed by the PAG and PMC, provided descending activation of the SPN via excitation of the dorsal interneuron. The PAG/PMC “switched on” and fired at a rate of 15 spikes/s when pelvic afferent firing, known to drive PMC activity (Blok and Holstege 2000), was high (>10 Hz) and the bladder volume exceeded the volume threshold for DECs. We included this descending excitatory pathway because it is necessary for the generation of DECs and interacts with the lumbosacral spinal circuits (Fowler et al. 2008; Sasaki and Sato 2013).
2.2 Linear integrate and fire (LIF) neurons
The model was implemented in Matlab (Mathworks, Natick, MA, USA) and was composed of 8 linear integrate and fire (LIF) neurons. The baseline firing rate of the pelvic afferents was ∼1 spikes/s when bladder volume was low, as described in electrophysiological experiments (Häbler et al. 1993). The other neurons were silent until activated and their firing rates were dependent on the integration of their respective synaptic inputs. Table 2 shows the equations used to model the network of LIF neurons and the parameters that were used in the model.
Table 2. Model equations and parameterization.
| Equations | Parameters |
|---|---|
| The linear integrate and fire neuron follows the form | Vrest = −65 mV |
| Vap = 60 mV | |
| Vthresh = −50 mV | |
| The dynamics of the neuron's membrane potential include contributions from membrane leak current, synaptic current and injected current to model adaptation. Using τm =CmRm, the equation can be rewritten as | Erev_ex = 0 mV |
| Erev_in = −80 mV | |
| dt = 0.1 ms | |
| The properties of the excitatory and inhibitory synaptic currents were defined by the parameters selected for gpeak, Erev, τrise and τdecay. A scaling factor, ḡ, controlled the relative weight of each synapse. | τm = 10 ms |
| Rm = 10 MΩ | |
| durrp = 1 ms | |
| The change in synaptic conductance following a spike was modeled by gsyn(t)=ḡsynf(e−(t−t0)/τdecay− e−(t−t0)/τrise), which was represented by coupled, linear ordinary differential equations (Roth and van Rossum 2009), | τr_ex = 0.9 ms |
| τd_ex = 12.15 ms | |
| τr_in = 1.1 ms | |
| τd_in = 10 ms | |
| gpeak_ex = 0.28 mS/cm2 | |
| gpeak_in = 1.5 mS/cm2 | |
| ωad0 = 0.1 | |
| Neuronal adaptation was modeled by increasing the voltage threshold of the neuron following each spike. The adaptation variable decayed with time constant ωad. | ωa_inc = 0.5 |
| τad = 35 ms | |
| When a spike fired in the presynaptic neuron, ωad was incremented by ωa_inc and the membrane potential was reset | ḡPel:INd = 0.45 |
| ḡPud:INd = 0.6 | |
| ḡPud:INm+ = 0.44 | |
| Simulated bladder pressure (PB) was recurrently ḡ calculated using functions of bladder volume at the previous time step and SPN output firing rate in a sliding window. The SPN firing rate calculation was generated from a polynomial fit of prior experimental results (Sasaki 1998). | ḡPud:INm- = 0.7 |
| ḡINm-:SPN = 0.65 | |
| ḡINm+:SPN= 0.6 | |
| where, f(FRSPN)=2×10−3FRSPN3 − 3.3×10−2FRSPN2 + 1.8FRSPN − 0.5 and f(VolB) = (1.5VolB − 10). | |
| ḡINd:SPN = 0.8 | |
| ḡPMC:INd = 0.33 | |
| Pelvic afferent firing rate was calculated from a polynomial fit of PB from recordings of the mean low threshold pelvic afferent response (Sengupta and Gebhart 1994) and fed back into the model. Negative firing rates generated by the polynomial were fixed at 0 spikes per second. FRpel(t) = f(PB(t−1)) = −3×10−8 PB5 + 1×10−5 PB4 −1.5×10−3 PB3 +7.9×10−2 PB2 −0.6PB |
ḡFB:INd = 0.6 |
| ḡSPN:FB = 1.0 | |
| FRpel_init = 1 spikes/s | |
| FRpud = 0 spikes/s | |
| FRPMC = 15 spikes/s | |
| VolDEC=13 mL | |
| wl =1000 ms |
Although the selection and optimization of the model neuron parameters was based on studies of the electrophysiological properties of neurons and behavior of the network, there was no explicit biophysical representation in this model. When an action potential occurred in the presynaptic neuron, a change in membrane conductance was generated in the post-synaptic neuron, causing a change in transmembrane voltage. The transient change in conductance was specified for each neuron to match the properties of the specified neurotransmitter receptor type likely to be found in each cell group and modeled by coupled, linear ordinary differential equations (Roth and van Rossum 2009). Excitatory postsynaptic potentials were parameterized to model glutamatergic synapses with NMDA receptor kinetics. Glutamate is a primary neurotransmitter employed by the spinal cord, and the excitatory connections between interneurons and SPN neurons are mediated by NMDA receptors (de Groat et al. 1998). Conversely, inhibition of the SPN by PN stimulation is mediated by GABAA receptors (McGee et al. 2014), and inhibitory post-synaptic potentials were parameterized to model GABAA receptor kinetics.
An action potential was initiated in the postsynaptic neuron when the transmembrane voltage surpassed the specified threshold (i.e., when Vm ≥ Vthresh, the transmembrane voltage was transiently set to the peak transmembrane voltage during the action potential Vap before returning to Vrest). Spike-triggered adaptation of neuron firing rates, described in Table 2, was implemented to increase the threshold voltage following repeated firing of the neuron. The parameters describing each neuron and synapse were selected to generate realistic SPN neuron firing rates and reproduce behavior from pelvic nerve recordings (Satchell and Vaughan 1989).
2.3 Closed-loop calculation of bladder pressure and pelvic afferent firing rate
Bladder activity is dependent on both pelvic efferent (SPN) firing and the volume in the bladder (de Groat and Ryall 1969; Sasaki 1998; Mendez et al. 2013), and we incorporated both of these in the calculation of the equivalent bladder pressure. Bladder pressure was recurrently simulated using functions of bladder volume (the threshold volume for distension-evoked contractions was approximately 13 mL) and a polynomial fit of an approximation of the median response of bladder pressure and SPN neuron firing rate data recorded in α-chloralose anesthetized cats (Sasaki 1998). The pelvic afferent firing rate was continually updated based on the bladder pressure from the previous time step using a polynomial fit of low threshold pelvic afferent activity as a function of bladder pressure, calculated from a selected recording from low threshold pelvic afferents in pentobarbital-anesthetized rats (Sengupta and Gebhart 1994). The smooth FRpel was transformed into a spike train by firing the pelvic afferent neuron when the time since the last spike exceeded the prescribed interspike interval calculated from the instantaneous frequency at each time step. The functions used to calculate bladder pressure from the firing rate of the model SPN neuron and bladder volume, and to calculate pelvic afferent firing from bladder pressure are shown in Table 2.
2.4 Model evaluation of pudendal afferent stimulation
We applied a variety of pudendal afferent stimulation inputs and tested multiple bladder volumes between 0.5 and 1.5 times VolDEC to evaluate the function of the neural network model. We assessed the effects of simulated slow infusion of fluid into the bladder to determine whether the model reproduced realistic responses to bladder filling. We also studied the effects of stimulation of pudendal afferents at different frequencies on the firing rate of the model SPN neuron and bladder pressure. The change in mean bladder pressure evoked by stimulation was calculated for each stimulation period as the mean bladder pressure during stimulation minus the pre-stimulation mean pressure. Further, we applied temporal patterns of pudendal afferent stimulation to compare the effects of stimulation pattern on the size of bladder contractions evoked by the model to previous experimental data from patterned stimulation of the dorsal genital branch of the pudendal nerve in cats (McGee and Grill 2013) and quantified the behavior of each model neuron.
3 Results
We developed a computational model of the sacral spinal neural circuit mediating the pudendo-vesical reflex and used the model to investigate mechanisms underlying the frequency dependence of bladder responses evoked by pudendal afferent stimulation.
3.1 SPN neuron firing rate and bladder pressure during bladder filling
The firing rate of the model SPN neuron and the simulated bladder volume were used to calculate bladder pressure, which was compared directly to results from experimental studies. The model SPN neuron firing rate was modulated by interneuron activity, which was dependent upon the firing rates of the pelvic and pudendal afferents. The model SPN neuron exhibited both tonic and burst-like firing activity (Satchell and Vaughan 1989), and the firing rate and behavior (Fig. 3) were within physiological limits reported in previous studies (de Groat et al. 1982; Sasaki 1998).
Fig. 3.
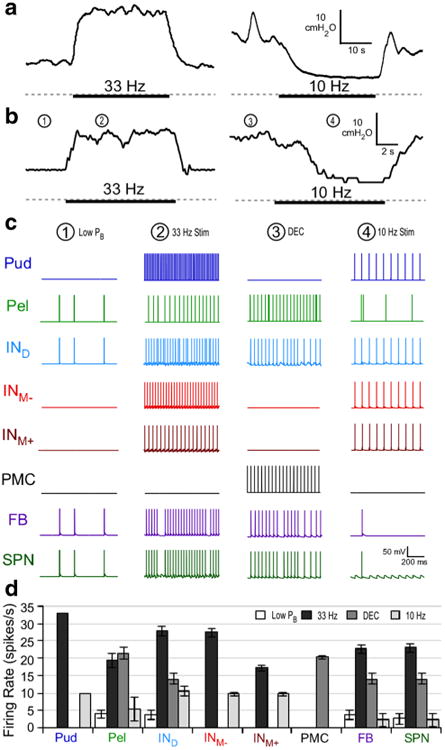
Reflex bladder excitation and inhibition by pudendal afferent stimulation. a Bladder pressure responses to pudendal afferent stimulation at 33 or 10 Hz in the α-chloralose-anesthetized cat from previous experiments (gray dashed line = 0 cmH2O) (McGee et al. 2014; McGee and Grill 2014). Heavy bar indicates when stimulation was applied. b Bladder pressure in response to stimulation of pudendal afferents in the model. c Model neuron activity at specific time points in B is shown. During times of low pressure (Low PB), the sacral parasympathetic nucleus (SPN) neuron firing remained low and irregular (1). During pudendal nerve stimulation at 33 Hz (2), the SPN neuron firing rate was regularized and increased by the excitatory interneurons, leading to an increase in bladder pressure. During distension-evoked contractions (DEC), SPN firing was increased, due to descending signals from the pontine micturition center (PMC) and the IND, producing high bladder pressures (3). 10 Hz PN stimulation (4) produced bladder inhibition and silenced SPN firing following a gradual decrease in firing rate. Inhibition occurred because hyperpolarization by INM- at this frequency blocked excitation of SPN by IND and INM+, leading to a reduction in bladder pressure. d Mean firing rate of each neuron under the four different conditions. Bars represent mean ± standard deviation for bladder pressures ranging from 0.6 to 0.95 VolDEC over 5 trials
Figure 2b shows the response of the neural network to a constant rate bladder infusion, which resulted in a DEC similar to what is seen with constant rate bladder infusion in the cat (Fig. 2a). The SPN neuron firing rate was sparse but burstlike at smaller bladder volumes and pressures, and increased slowly with volume during bladder filling. At larger bladder volumes, increased bladder pressure and pelvic afferent activity produced additional increases in SPN firing rate. Prior to the onset of a coordinated DEC, transient increases and decreases in bladder pressure were observed, corresponding to bursts and pauses in the SPN neuron firing rate. A DEC was triggered by the PAG/PMC when bladder volume and pelvic afferent activity exceeded threshold. Above the DEC threshold volume, the pressure–time profile of bladder contractions mimicked those seen in vivo (Fig. 2), and the SPN neuron fired at ∼15 spikes/s (Fig. 3d).
Fig. 2.
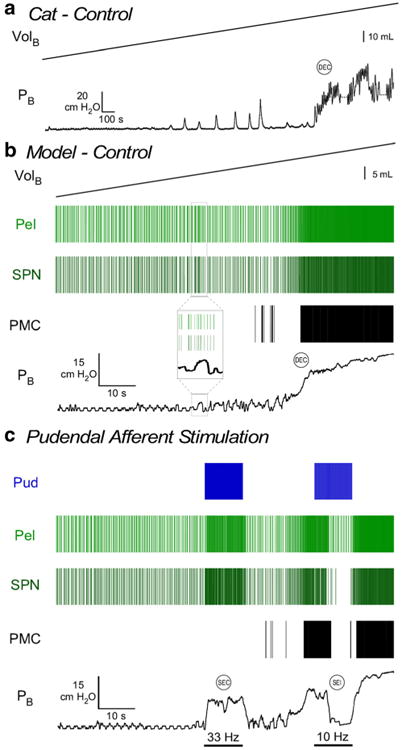
Behavior in response to bladder filling and pudendal afferent stimulatio. a Bladder pressure during slow, constant rate bladder filling in the adult cat anesthetized with α-chloralose (McGee et al. 2014). Bladder pressure slowly increases until a distension-evoked contraction (DEC) is evoked. Transient non-voiding contractions were present before the DEC. b Neuronal spiking activity is represented with rasters of the model pudendal afferents (Pud), pelvic afferents (Pel), and sacral parasympathetic nucleus (SPN) neuron. Increasing the model bladder volume (0 to approximately 1.5 times VolDEC; used in calculation of pelvic afferent firing rate) produced a gradual increase in bladder pressure until a DEC was evoked, via the PMC, wherein bladder volume and pelvic afferent activity are high. Transient changes in bladder pressure that were produced by burst-like activity of the SPN neuron preceded the coordinated bladder contraction (inset) and mimicked non-voiding contractions seen in vivo. c 33 Hz stimulation of pudendal afferents evoked a stimulation-evoked bladder contraction (SEC) coincident with an increase in SPN neuron firing rate. 10 Hz stimulation of pudendal afferents produced stimulation-evoked inhibition (SEI) of DECs coincident with silencing of SPN neuron firing
3.2 Model reproduces in vivo responses to pudendal afferent stimulation
The response of the neural network to different frequencies of pudendal afferent stimulation was simulated to investigate reflex activation or inhibition of the bladder. The synaptic weights of model neurons in the network model were adjusted to reproduce the tuning curve of bladder excitation across frequencies of pudendal afferent stimulation (Fig. 4). Similar to what is observed experimentally with stimulation of the dorsal genital nerve (DGN) or the dorsal genital pathway of the PN (Boggs et al. 2006; Yoo et al. 2008), for a range of bladder volumes, high frequencies of stimulation evoked bladder contractions, but frequencies above 33 Hz were less effective than 33 Hz. Further, stimulation at frequencies of 2– 20 Hz failed to evoke robust bladder contractions and 10 Hz stimulation produced a reduction in bladder pressure. Bladder inhibition by stimulation applied during DECs was also frequency dependent, as stimulation at 10 Hz produced robust inhibition, and a small range of frequencies, i.e., 5-15Hz, also produced some degree of inhibition.
Fig. 4.
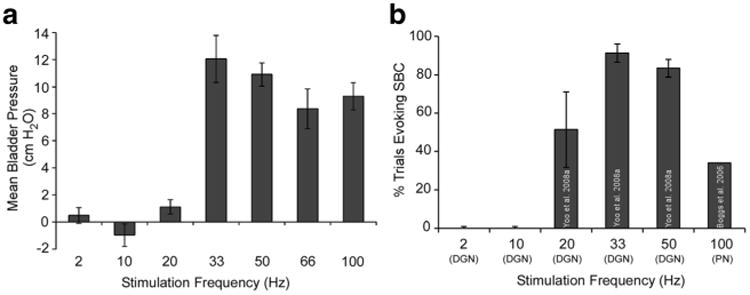
Stimulation frequency dependent activation of the bladder in the model matched experimental results. a Change in bladder pressure during stimulation, relative to baseline, varied with the frequency of pudendal afferent stimulation in the model. Bars represent mean ± standard deviation for bladder pressures ranging from 0.6 to 0.85 VolDEC over ten trials. b Percentage of trials during which a sustained bladder contraction (SBC) was evoked across different frequencies of pudendal (dorsal genital nerve, DGN) afferent stimulation in α-chloralose-anesthetized cats (Boggs et al. 2006; Yoo et al. 2008)
Further, the size of bladder contractions evoked by stimulation of pudendal afferents was dependent on bladder volume. Pudendal afferent stimulation at 33 Hz failed to evoke robust bladder contractions (mean bladder pressure >10 cmH2O) below ∼70 % of the volume necessary to evoke DECs, a hallmark of the pudendo-vesical reflex. The firing rate of the SPN neuron and bladder pressure evoked by 33 Hz stimulation increased with increases in bladder volume. Figure 2c shows an example where 33 Hz pudendal afferent stimulation produced an increase in SPN neuron firing rate and bladder pressure during filling (prior to the initiation of DECs), while 10 Hz pudendal afferent stimulation produced a decrease in SPN neuron firing rate and a reduction in bladder pressure, consistent with experimental results (Woock et al. 2008; Woock et al. 2011). Isovolumetric bladder contractions evoked by 33 Hz pudendal afferent stimulation and inhibition of isovolumetric DECs by 10 Hz pudendal afferent stimulation also mimicked results from pudendal afferent stimulation under isovolumetric bladder conditions in the cat (McGee et al. 2014; McGee and Grill 2014) (Fig. 3a–b).
Changes in bladder pressure during stimulation of the pudendal afferents in the model were a product of changes in SPN neuron firing rate. For example, during a robust bladder contraction evoked by 33 Hz pudendal afferent stimulation, the SPN neuron firing rate increased from ∼3 to ∼22 Hz. The changes in firing rate of all model neurons in response to 10 or 33 Hz pudendal afferent stimulation are shown in Fig. 3c–d. During 33 Hz pudendal afferent stimulation, the SPN neuron firing rate was increased and regularized by an increase in the firing rate of the excitatory interneurons. During 10 Hz pudendal afferent stimulation, synchronous firing by the excitatory and inhibitory interneurons silenced the SPN neuron, i.e., the medial inhibitory interneuron consistently fired at the same time as the excitatory interneurons, which prevented activation of SPN and produced a decrease in bladder pressure (Fig. 3b). In contrast, 33 Hz pudendal afferent stimulation produced an increase in SPN activation as a result of asynchronous interneuron firing, as a result of neural adaptation and differences in post-synaptic potentials across the interneurons. Figure 5a–c demonstrate the critical importance of the shape of post-synaptic potentials and spike arrival times, which together produced the frequency-dependent effects of pudendal afferent stimulation.
Fig. 5.
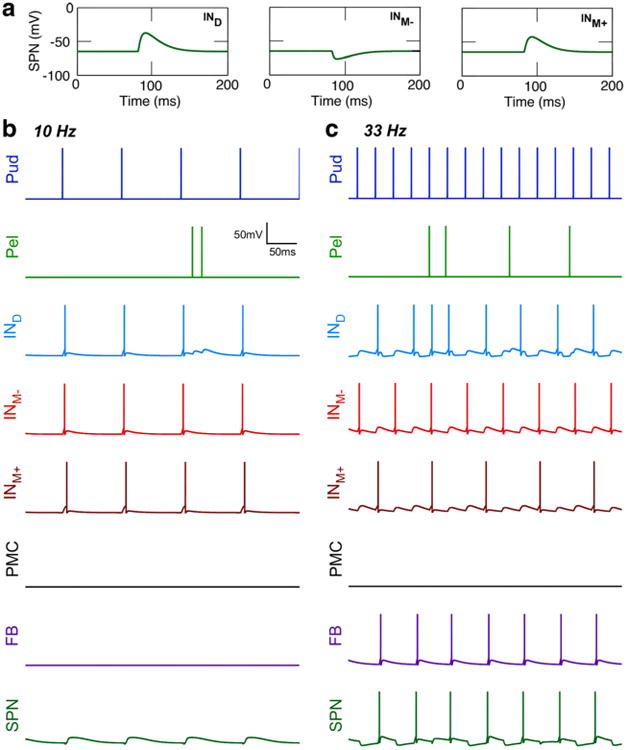
Interneuron firing activity drives frequency-dependent effects of pudendal afferent stimulation on SPN firing. a Post-synaptic potentials recorded in the SPN neuron transmembrane voltage (Vm) following the firing of a spike in each of the model interneurons, IND (left), INM-(middle), and INM+ (right). b Firing activity of model neurons in response to 10 Hz pudendal stimulation. The synchronized firing of excitatory and inhibitory interneurons at 10 Hz inhibited activation of SPN. c Firing activity of model neurons in response to 33 Hz pudendal stimulation. SPN activation resulted from asynchronous firing of the interneurons due to adaptation and differences in the shapes of post-synaptic potentials
3.3 Network structure mediates frequency and bladder volume dependent effects of stimulation
To validate the structure of the network model and evaluate potential mechanisms underlying the frequency-dependent effects of PN stimulation on the bladder, we removed the contribution of the various interneurons and evaluated bladder responses to different frequencies of pudendal afferent stimulation. Removing the medial interneurons, INM+ and INM-, produced an increase in neural activity throughout the network and monotonically increasing bladder pressure with increasing stimulation frequency (Fig. 6a). Further exploration of the model parameter space could not resolve the inability of this topology to reproduce the frequency-dependent effects of stimulation. Thus, neither inhibition with 10 Hz stimulation, nor a decline in the evoked bladder pressure at stimulation frequencies >40 Hz was observed when the medial interneurons were removed, revealing that these interneurons are critical and feedback inhibition alone was not sufficient to produce the frequency-dependent effects of stimulation.
Fig. 6.
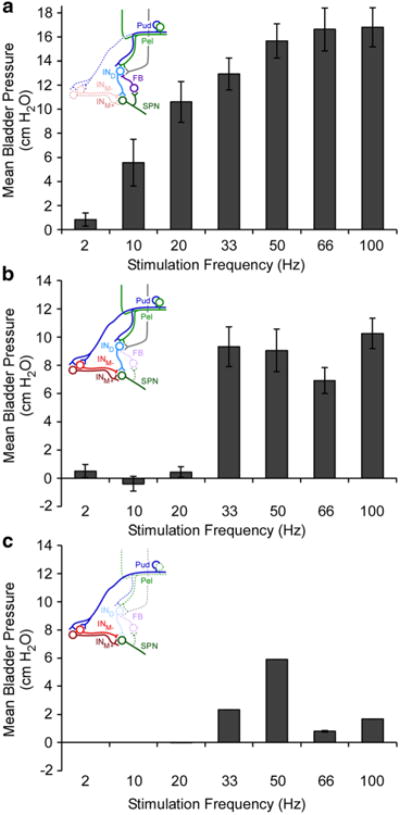
Stimulation frequency dependent responses in model variations. a Removal of the medial interneurons (INM+ and INM-) produced a thresholded monotonic increase in normalized bladder pressure with stimulation frequency that is not seen in experimental studies. b Removal of the inhibitory feedback interneuron (FB) did not substantially alter the effects of stimulation on the size of stimulation-evoked bladder contractions, but increased the mean pressure evoked with high frequency stimulation. c Reduction of the synaptic weight (0.8 → 0.2) of the dorsal interneuron (IND) produced frequency-dependent responses to stimulation, but robust bladder contractions were not evoked due to the removal of contributions from pelvic afferents. Bars represent mean ± standard deviation for bladder pressures ranging from 0.6 to 0.85 VolDEC over ten trials
We also evaluated the effects of removing the feedback (FB) interneuron, or dorsal interneuron, IND, from the network to determine their contributions to the frequency-dependent effects of pudendal afferent stimulation on bladder pressure and SPN neuron firing rate. The firing rate of the SPN neuron and bladder pressure during stimulation at most frequencies were not substantially different following elimination of the FB interneuron from the network (Fig. 6b). However, without feedback inhibition on IND, the SPN neuron fired at a higher rate in response to high frequency inputs, which led to an increase in bladder pressure with 100 Hz stimulation compared to the intact network.
To determine the role of the IND, we removed or reduced the synaptic weight (0.8 → 0.2) of the IND. The frequency-dependent excitation of the SPN neuron and consequent increases in bladder pressure with pudendal afferent stimulation remained intact (Fig. 6c), but the volume-dependent effects of stimulation and pelvic afferent contribution to SPN firing rate were lost. Pudendal afferent stimulation above 33 Hz caused the SPN neuron to fire, but the rate was too low to evoke robust contractions. Stimulation frequencies between 2 and 20 Hz and above 66 Hz failed to evoke SPN neuron firing. This revealed that the IND was not necessary to produce the stimulation frequency-dependent changes in SPN neuron firing rate or bladder pressure, but was critical to reproduce the volume-dependence of robust stimulation-evoked bladder contractions.
3.4 Blockade of GABAergic activity abolished bladder inhibition by pudendal afferent stimulation
Bladder inhibition by low frequency pudendal afferent stimulation is mediated by GABAAergic mechanisms in the spinal cord (McGee et al. 2014), and we simulated GABAergic blockade by reducing the synaptic weights of the inhibitory medial interneuron (0.65 → 0.2) and feedback interneuron (0.6 → 0.2). Similar to what was seen experimentally following blockade of GABAA receptors with picrotoxin (McGee et al. 2014), bladder inhibition evoked by 10 Hz pudendal afferent stimulation was abolished following blockade of the inhibitory interneurons (Fig. 7). The inhibitory medial inter-neuron (INM-) was most important for the bladder inhibition evoked by 10 Hz stimulation, as inhibition persisted when only the FB interneuron was removed from the network. Bladder excitation by 33 Hz pudendal afferent stimulation remained intact following blockade of the inhibitory interneurons, as observed experimentally (Fig. 7).
Fig. 7.
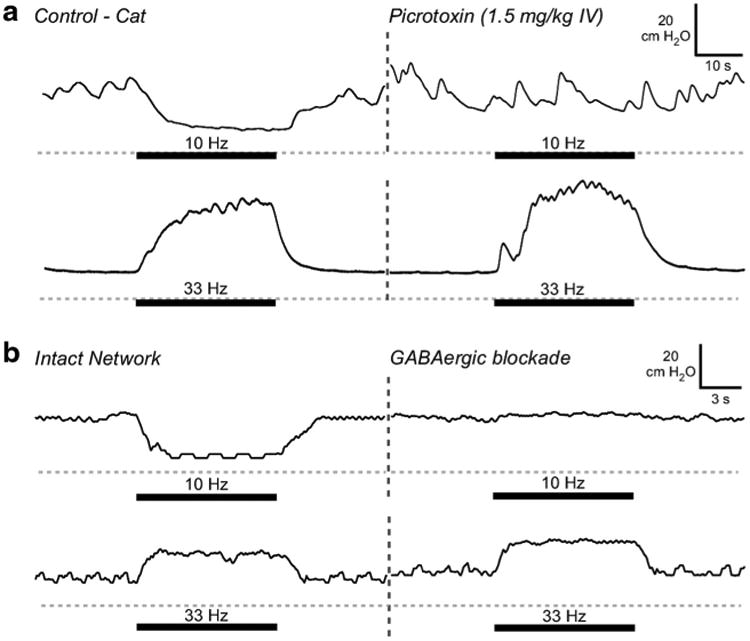
Effects of gabaergic blockade on response to pudendal afferent stimulation. a Bladder pressure traces from experiment in α-chloralose anesthetized adult cat (gray dashed line = 0 cmH2O) (McGee et al. 2014; McGee and Grill 2014). Thick bar indicates when stimulation was applied. Inhibition of bladder contractions by 10 Hz pudendal DGN afferent stimulation was eliminated following administration of the GABAA receptor antagonist picrotoxin. Stimulation-evoked bladder contractions were produced by 33 Hz stimulation before and after the administration of picrotoxin. b Simulated blockade of GABAergic inhibitory synapses in the model mimicked the effects of administration of picrotoxin. Pudendal afferent stimulation-evoked inhibition with 10 Hz was lost following blockade of model GABAergic synapses. Bladder contractions evoked by 33 Hz pudendal afferent stimulation were unaffected by the reduction in synaptic weight of inhibitory synapses in the neural network, consistent with experimental results (McGee et al. 2014)
3.5 Response to temporal patterns of pudendal afferent stimulation
We evaluated the effects of temporal patterns of pudendal afferent stimulation, which produced different bladder responses in experiments in cats (McGee and Grill 2013; McGee and Grill 2015), on the firing rates of neurons in our validated model. As in the experiments, bladder activation differed across patterns of pudendal afferent stimulation (Fig. 8). Patterns that featured small changes in inter-pulse-interval (IPI) over time (Pattern #2, #3, #6 or #7) produced bladder contractions and SPN firing rates comparable to Pattern #1. Temporal patterns of stimulation that contained pauses, bursts, or random trains of stimulation produced bladder contractions with smaller mean bladder pressures than 33 Hz stimulation, as also observed experimentally. These smaller bladder contractions were mediated by changes in interneuron firing rate during pauses or bursts that produced a reduction in SPN neuron firing rate (Fig. 9). Pauses in stimulation in Pattern #5 or Pattern #8 silenced the interneurons and interrupted the excitatory activation of the SPN, causing a decrease in SPN firing rate and bladder pressure. During bursts of high frequency stimulation (Pattern #4, #5 or #9), the interneurons failed to increase their firing rate to match the input, and dominance by the inhibitory interneuron (INM-) prevented the SPN from firing at a high rate. Interestingly, Pattern #9 (alternating 10 and 20 ms IPIs) evoked smaller mean bladder pressures than either 66 Hz (15 ms) or 100 Hz (10 ms) stimulation, indicating that the pattern of pulse presentation was also critical in determining response to stimulation. The changing IPIs in Pattern #9 triggered INM- to block excitation by IND, demonstrating that pattern, in addition to frequency, influences the response of the neural network to PN stimulation.
Fig. 8.
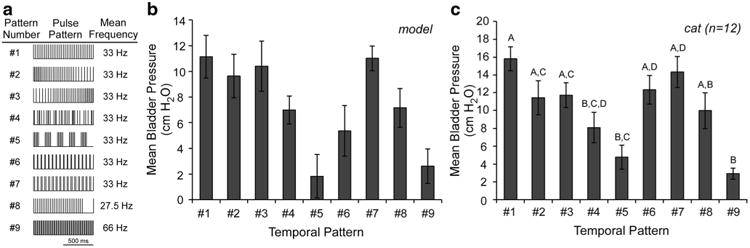
Bladder reponses evoked by different temporal patterns of pudendal afferent stimulation. a Temporal patterns of stimulation applied to the model and in experiments (McGee and Grill 2015). Pattern #1 was a regular frequency train with a mean of 33 Hz. Patterns #2 and #3 were decreasing and increasing ramp trains, respectively, where the inter-pulse-interval (IPI) changed gradually at each pulse (mean = 33 Hz). Pattern #4 was a random train from a uniform random distribution (IPIs were limited between 2 and 100 ms), with mean rate of 33 Hz. Pattern #5 consisted of repeating 100 ms periods of either 66 Hz stimulation or no stimulation, for an overall mean rate of 33 Hz. Patterns #6 and #7 were patterns of stimulation with alternating IPI presentation for an overall mean rate of 33 Hz, 10 and 50 ms IPIs and 20 and 40 ms IPIs, respectively. Pattern #8 was 1000 s of 33 Hz stimulation followed by a 200 ms pause for an effective stimulation rate of 27.5 Hz. Pattern #9 was inspired by a previous study (Bruns et al. 2008), and contained two pulses of 100 Hz stimulation repeated at 33 Hz, for a mean rate of 66 Hz. b Bladder pressure evoked by stimulation of pudendal afferents in the model varied according to temporal pattern applied. Bars represent mean ± standard deviation for bladder pressures ranging from 0.6 to 0.85 VolDEC over ten trials. c Bladder pressure evoked by different temporal patterns of pudendal afferent (DGN) in α-chloralose-anesthetized adult cats (n = 12) (McGee and Grill 2015)
Fig. 9.
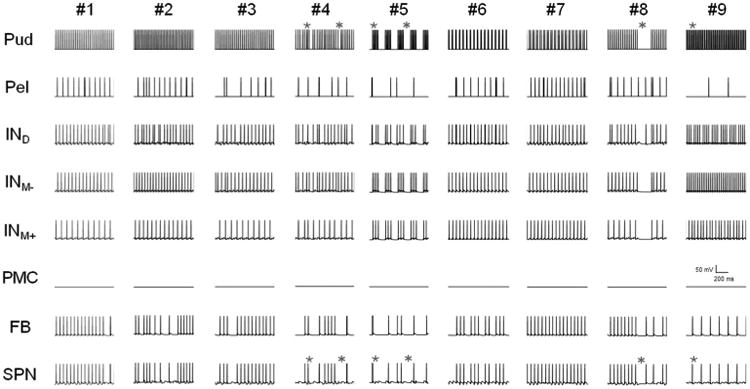
Model neuron firing varies with the temporal pattern of stimulation. Firing activity of the model neurons is shown for 1 s of stimulation with each pattern of stimulation (Patterns #1 to #9). Each row represents the firing activity of each model neuron over time. Stimulation with pauses and high frequency bursts, Pattern #4, #5, #8, #9, produced SPN firing rates that were lower than regular stimulation (Pattern #1). Patterns with small changes in stimulation pattern (#2, #3, #6, and #7) produced SPN firing rates similar to Pattern #1. During pauses (*), the interneurons' firing rates decreased, causing the SPN to stop firing. During bursts of high frequency stimulation (★), the interneurons failed to increase their firing rate to match the input, and dominance by the inhibitory interneuron (INM-) prevented the SPN from firing at a high rate. These examples demonstrate that certain key features of patterned stimulation trains (e.g., bursts, pauses), via dynamic changes in interneuron firing rates, can modulate SPN firing rate and bladder pressure
4 Discussion
We implemented and validated a computational model of the spinal neural network that mediates the effects of pudendal afferent stimulation on the bladder. The model reproduced the effects of pudendal afferent stimulation frequency and pattern measured experimentally, and allowed exploration of the mechanisms underlying the strong stimulation frequency-dependent effects. Although the model included a simple representation of the descending pathway from the PAG/PMC to the SPN, the frequency and pattern-dependent effects of pudendal afferent stimulation were determined by changes in firing rate of spinal interneurons, suggesting that neural network interactions at the sacral level can mediate the bladder response to different frequencies or temporal patterns of pudendal afferent stimulation. Inclusion of more neurons (e.g., multiple pelvic afferents with varied low-threshold and/or high-threshold responses to bladder distension) or additional neural pathways (e.g., the cranial sensory pathway of the pudendal nerve) in a future version of the model would improve the realism of the model and enable further exploration of the mechanisms of the frequency-dependent effects of stimulation. Furthermore, improving the biophysical representation of the model may also permit the demonstration of features that are not encompassed by this model of stimulation of the dorsal genital pathway of the pudendal nerve and resulting pudendo-vesical reflexes.
4.1 Model bladder pressure and firing rate
Simulated bladder filling produced realistic changes in SPN firing rate and bladder pressure, eventually triggering a DEC (Fig. 2b). At small volumes, SPN firing rate and bladder pressure were low. Transient increases and decreases in bladder pressure were observed at volumes and bladder pressures below the threshold for DECs, mimicking non-voiding contractions seen in cystometric studies (Hashim and Abrams 2006). These non-voiding contractions resulted from irregular, burst-like activity from the network processing of afferents that failed to produce a sustained increase in the firing rates of the interneurons or SPN neuron. At larger volumes and higher bladder pressures, continuous descending excitation from PAG/PMC, combined with increased pelvic afferent activity, produced DECs in the model. This matched a previous experimental study where DECs were evoked only by electrical stimulation of the PMC at high bladder pressures via polysynaptic excitation of SPN neurons (Sasaki and Sato 2013). The pressure–time profile of distension-evoked and stimulation-evoked bladder contractions in the model exhibited transient increases and decreases in pressure (Fig. 2), which corresponded to brief changes in the firing rate of the SPN neuron, although the time scale of these changes in bladder pressure in the model were shorter than those observed in vivo. Bursting by preganglionic SPN neurons corresponded to rhythmic bladder activity in vivo (de Groat et al. 1982). Our model revealed that these bursts and pauses in the SPN firing rate were the product of competing excitation and inhibition of the SPN neuron by the network interneurons.
4.2 Model reproduced responses to pudendal afferent stimulation
Our model reproduced the hallmarks of the pudendo-vesical reflex: volume-dependence of stimulation-evoked bladder contractions and stimulation frequency-dependent bladder activation. Pudendal afferent stimulation at 33 Hz during low bladder volumes (<∼70 % DEC volume threshold) failed to evoke robust bladder contractions, and the size of stimulation-evoked bladder contractions increased with bladder volume. Previous studies suggested that the volume-dependence of pudendal afferent stimulation-evoked bladder contractions was the result of a convergence of pudendal and pelvic afferent inputs (Woock et al. 2011). Manipulation of the model corroborates this suggestion, as the IND, which receives both pelvic and pudendal afferent inputs, mediated the volume-dependence.
The bladder response evoked by pudendal afferent stimulation in the model exhibited a strong dependence on the frequency of stimulation. The properties of the model were selected to reproduce to available results from in vivo studies (primarily cats, but included results from rats and monkeys) and the selection of different model species or experimental methods, (e.g., different anesthesia regimens), may alter the final network properties. In the cat, 33 Hz stimulation evokes robust bladder contractions and augments ongoing bladder contractions, while 10 Hz stimulation inhibits bladder contractions (Woock et al. 2008); the synaptic weights of neurons in the model were manually adjusted to reproduce these frequency-dependent responses and fixed for the remainder of the analysis. High frequency stimulation (33 Hz) evoked large bladder contractions that were sustained during the period of stimulation, caused by an increase in SPN neuron firing rate that was driven by increased excitatory medial and dorsal interneuron activity. Low frequency (10 Hz) pudendal afferent stimulation did not evoke bladder contractions and inhibited ongoing bladder contractions, due to firing of the inhibitory medial interneuron and silencing of SPN neuron activity.
Stimulation from 2 to 20 Hz was not as effective at evoking bladder contractions as 33 Hz (Boggs et al. 2006; Yoo et al. 2008). In previous experiments, responses to 20 Hz stimulation were variable, producing bladder contractions approximately 54 % of the time. In the model, 20 Hz stimulation did not evoke bladder contractions; however a slightly higher frequency (25 Hz) did evoke bladder contractions, consistent with a frequency threshold between 20 and 25 Hz. Frequencies higher than 33 Hz evoked robust bladder contractions in the model, but the amplitude of the bladder contractions did not exceed that evoked by 33 Hz stimulation, producing a peak in the effect of pudendal afferent stimulation at approximately 33 Hz that matched experimental results (Yoo et al. 2008). These results indicate that the structure of the lumbosacral spinal network is necessary and sufficient to account for the frequency-dependent effects of pudendal afferent stimulation on bladder activity, as suggested by animal studies where pudendal afferent stimulation evoked robust bladder contractions and inhibition following acute or chronic SCI (Tai et al. 2006; Woock et al. 2008; Xiao et al. 2014).
Further, the model reproduced the bladder response to specific and widely differing temporal patterns of pudendal afferent stimulation (McGee and Grill 2013; McGee and Grill 2015). Temporal patterns that featured small changes in IPI did not produce substantial differences in interneuron or SPN firing rates compared to regular 33 Hz stimulation (Fig. 9). Patterns that included pauses and high frequency bursts were ineffective at increasing SPN firing rate in the model and did not evoke bladder contractions in the experiments. The pauses interrupted the interneuron firing and silenced the SPN neuron, while the bursts of high frequency (66 Hz) stimulation periods failed to increase the SPN firing rate to follow the high input rate, producing an ineffective stimulus train despite the mean train rate of 33 Hz. Randomly patterned stimulation with a mean rate of 33 Hz, was less effective than regular 33 Hz stimulation in the model, matching experimental results. This decreased effectiveness was caused by the random bursts and pauses of stimulation that interrupted the regular firing of model interneurons and decreased the SPN firing rate (Fig. 9), resulting in smaller and less consistent bladder contractions.
4.3 Mechanisms of sacral processing of pudendal afferent stimulation
The model enabled manipulation of the neural network to study the underlying mechanisms of the frequency-dependent effects of pudendal afferent stimulation. Removing the contribution of the medial interneurons resulted in a simple network with a single inhibitory feedback neuron that produced a response to different frequencies of pudendal afferent stimulation inconsistent with experimental results. This suggests that the excitatory and inhibitory medial interneuron contributions were critical to the frequency-dependent effects of stimulation on bladder activity. Further, the respective spike timing of these medial interneurons was found to be critical to the pattern- and frequency-dependent effects of stimulation, as the spike timing (i.e., synchronous firing at low frequencies, which produced inhibition of SPN and asynchronous firing at higher frequencies which enabled activation of SPN) was mediated by neural adaptation and the size and shape of post-synaptic potentials. Thus, the structure of this network and the programmed synaptic properties for excitatory and inhibitory interneurons, particularly the medial interneurons, mediate the effects of pudendal afferent stimulation on SPN firing rate and bladder pressure.
The model also reproduced the bladder response to pudendal afferent stimulation following selective pharmacological blockade of GABAA, simulated by reducing the strength of model GABAergic synapses. Picrotoxin reversibly blocked 10 Hz pudendal afferent stimulation-evoked inhibition in cats (McGee et al. 2014) and in the model, bladder inhibition was abolished after blockade of the inhibitory synapses. Instead, slight bladder excitation was seen in response to 10 Hz stimulation, which matched the in vivo results. Bladder excitation by 33 Hz stimulation was preserved following picrotoxin in the cat (McGee et al. 2014), and in the model, bladder excitation by 33 Hz pudendal afferent stimulation persisted following simulated blockade of GABAergic synapses.
The anatomical structure of the model of excitatory and inhibitory interneurons in the network was necessary and sufficient to reproduce both the volume-dependence of stimulation-evoked bladder contractions and frequency-dependence of stimulation on bladder activity. The frequency and pattern-dependent effects of pudendal afferent stimulation were determined by changes in firing rate of spinal interneurons, suggesting that neural network interactions at the lumbosacral level can mediate the bladder response to different frequencies or temporal patterns of pudendal afferent stimulation.
Acknowledgments
This work was funded by National Institutes of Health (NIH) R01 NS050514.
Footnotes
Compliance with ethical standards: Conflict of interest The authors declare that they have no conflict of interest.
References
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourology and Urodynamics. 2002;21(2):167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. Journal of Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. The Journal of Neuroscience. 1997;17(21):8402–8407. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaanssen EH, van Leeuwen JL, Vanderschoot J, Redert PA. A myocybernetic model of the lower urinary tract. Journal of Theoretical Biology. 1996;178(2):113–133. doi: 10.1006/jtbi.1996.0011. [DOI] [PubMed] [Google Scholar]
- Blok BFM, Holstege G. The pontine micturition center in rat receives direct lumbosacral input. An ultrastructural study. Neuroscience Letters. 2000;282(1–2):29–32. doi: 10.1016/s0304-3940(00)00833-8. [DOI] [PubMed] [Google Scholar]
- Blok BFM, van Maarseveen JTPW, Holstege G. Electrical stimulation of the sacral dorsal gray commissure evokes relaxation of the external urethral sphincter in the cat. Neuroscience Letters. 1998;249(1):68–70. doi: 10.1016/s0304-3940(98)00382-6. [DOI] [PubMed] [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. Journal of Physiology. 2006;577(1):115–126. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns TM, Bhadra N, Gustafson KJ. Variable patterned pudendal nerve stimuli improves reflex bladder activation. IEEE Transactions on Rehabilitation Engineering. 2008;16(2):140–148. doi: 10.1109/TNSRE.2007.914460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC. Mechanisms underlying recurrent inhibition in the sacral parasympathetic outflow to the urinary bladder. Journal of Physiology. 1976;257(2):503–513. doi: 10.1113/jphysiol.1976.sp011381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Ryall RW. Recurrent inhibition in sacral parasympathetic pathways to the bladder. The Journal of Physiology. 1968;196(3):579–591. doi: 10.1113/jphysiol.1968.sp008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Ryall RW. Reflexes to sacral parasympa-thetic neurones concerned with micturition in the cat. Journal of Physiology. 1969;200(1):87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Wickens C. Organization of the neural switching circuitry underlying reflex micturition. Acta Physiologica. 2013;207(1):66–84. doi: 10.1111/apha.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Booth AM, Milne RJ, Roppolo JR. Parasympathetic preganglionic neurons in the sacral spinal cord. Journal of the Autonomic Nervous System. 1982;5(1):23–43. doi: 10.1016/0165-1838(82)90087-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behavioural Brain Research. 1998;92(2):127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Comparative Physiology. 2015;5(1):327–396. doi: 10.1002/cphy.c130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nature Reviews Neuroscience. 2008;9(6):453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill WM, Bhadra N, Wang B. Bladder and urethral pressures evoked by microstimulation of the sacral spinal cord in cats. Brain Research. 1999;836(1):19–30. doi: 10.1016/s0006-8993(99)01581-4. [DOI] [PubMed] [Google Scholar]
- Häbler HJ, Jänig W, Koltzenburg M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. The Journal of Physiology. 1993;463(1):449–460. doi: 10.1113/jphysiol.1993.sp019604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim H, Abrams P. Is the bladder a reliable witness for predicting detrusor overactivity? Journal of Urology. 2006;175(1):191–194. doi: 10.1016/S0022-5347(05)00067-4. discussion 194–195. [DOI] [PubMed] [Google Scholar]
- Hosein RA, Griffiths DJ. Computer simulation of the neural control of bladder and urethra. Neurourology and Urodynamics. 1990;9(6):601–618. [Google Scholar]
- Jilge B, Minassian K, Rattay F, Dimitrijevic MR. Frequency-dependent selection of alternative spinal pathways with common periodic sensory input. Biological Cybernetics. 2004;91(6):359–376. doi: 10.1007/s00422-004-0511-5. [DOI] [PubMed] [Google Scholar]
- Ku JH. The management of neurogenic bladder and quality of life in spinal cord injury. BJU International. 2006;98(4):739–745. doi: 10.1111/j.1464-410X.2006.06395.x. [DOI] [PubMed] [Google Scholar]
- McGee MJ, Grill WM. Temporal patterns of pudendal afferent stimulation modulate reflex bladder activation. Neural Engineering (NER) 2013 6th International IEEE/EMBS Conference on 2013 [Google Scholar]
- McGee MJ, Grill WM. Selective co-stimulation of pudendal afferents enhances bladder activation and improves voiding efficiency. Neurourology and Urodynamics. 2014;33(8):1272–1278. doi: 10.1002/nau.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee MJ, Grill WM. Temporal pattern of stimulation modulates reflex bladder activation by pudendal nerve stimulation. Neurourol Urodyn. 2015 doi: 10.1002/nau.22822. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee MJ, Danziger ZC, Bamford JA, Grill WM. A spinal GABAergic mechanism is necessary for bladder inhibition by pudendal afferent stimulation. American Journal of Physiology Renal Physiology. 2014;307(8):F921–930. doi: 10.1152/ajprenal.00330.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez A, Sawan M, Minagawa T, Wyndaele JJ. Estimation of bladder volume from afferent neural activity. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2013;21(5):704–715. doi: 10.1109/TNSRE.2013.2266899. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. Journal of Comparative Neurology. 1984;226(2):238–245. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Roppolo J, Morgan C, de Groat WC. Parasympathetic preganglionic neurons and visceral primary afferents in monkey sacral spinal cord revealed following application of horseradish peroxidase to pelvic nerve. The Journal of Comparative Neurology. 1983;216(1):36–52. doi: 10.1002/cne.902160105. [DOI] [PubMed] [Google Scholar]
- Peng CW, Chen JJ, Cheng CL, Grill WM. Improved bladder emptying in urinary retention by electrical stimulation of pudendal afferents. Journal of Neural Engineering. 2008;5(2):144–154. doi: 10.1088/1741-2560/5/2/005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikov V, Bullara L, McCreery DB. Intraspinal stimulation for bladder voiding in cats before and after chronic spinal cord injury. Journal of Neural Engineering. 2007;4(4):356. doi: 10.1088/1741-2560/4/4/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppolo JR, Nadelhaft I, De Groat WC. The organization of pudendal motoneurons and primary afferent projections in the spinal cord of the rhesus monkey revealed by horseradish peroxidase. The Journal of Comparative Neurology. 1985;234(4):475–488. doi: 10.1002/cne.902340406. [DOI] [PubMed] [Google Scholar]
- Roth A, van Rossum MC. 6Modeling Synapses 2009 [Google Scholar]
- Sasaki M. Bladder motility and efferent nerve activity during isotonic and isovolumic recording in the cat. Journal of Physiology. 1998;510(Pt 1):297–308. doi: 10.1111/j.1469-7793.1998.297bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Sato H. Polysynaptic connections between Barrington's nucleus and sacral preganglionic neurons. Neuroscience Research. 2013;75(2):150–156. doi: 10.1016/j.neures.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Satchell P, Vaughan C. Efferent pelvic nerve activity, ganglionic filtering, and the feline bladder. American Journal of Physiology. 1989;256(6 Pt 2):R1269–1273. doi: 10.1152/ajpregu.1989.256.6.R1269. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. Journal of Neurophysiology. 1994;72(5):2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- Shafik A, Shafik AA, El-Sibai O, Ahmed I. Role of positive urethrovesical feedback in vesical evacuation. The concept of a second micturition reflex: the urethrovesical reflex. World Journal of Urology. 2003;21(3):167–170. doi: 10.1007/s00345-003-0340-5. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ. Sacral spinal interneurones and the control of urinary bladder and urethral striated sphincter muscle function. The Journal of Physiology. 2001;533(1):57–63. doi: 10.1111/j.1469-7793.2001.0057b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Experimental Neurology. 2006;197(1):225–234. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Thor KB, Morgan C, Nadelhaft I, Houston M, de Groat WC. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. The Journal of Comparative Neurology. 1989;288(2):263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- van Duin F, Rosier PF, Bemelmans BL, Debruyne FM, Wijkstra H. A computer model for describing the effect of urethral afferents on simulated lower urinary tract function. Archives of Physiology and Biochemistry. 1999;107(3):223–235. doi: 10.1076/apab.107.3.223.4333. [DOI] [PubMed] [Google Scholar]
- Woock J, Yoo P, Grill W. Activation and inhibition of the micturition reflex by penile afferents in the cat. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2008;294(6):R1880–1889. doi: 10.1152/ajpregu.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woock JP, Yoo PB, Grill WM. Mechanisms of reflex bladder activation by pudendal afferents. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011;300(2):R398–R407. doi: 10.1152/ajpregu.00154.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Rogers MJ, Shen B, Wang J, Schwen Z, Roppolo JR, de Groat WC, Tai C. Somatic modulation of spinal reflex bladder activity mediated by nociceptive bladder afferent nerve fibers in cats. American Journal of Physiology - Renal Physiology. 2014 doi: 10.1152/ajprenal.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo PB, Grill WM. Minimally-invasive electrical stimulation of the pudendal nerve: a pre-clinical study for neural control of the lower urinary tract. Neurourology and Urodynamics. 2007;26(4):562–569. doi: 10.1002/nau.20376. [DOI] [PubMed] [Google Scholar]
- Yoo P, Klein S, Grafstein N, Horvath E, Amundsen C, Webster G, Grill W. Pudendal nerve stimulation evokes reflex bladder contractions in persons with chronic spinal cord injury. Neurourology and Urodynamics. 2007;26(7):1020–1023. doi: 10.1002/nau.20441. [DOI] [PubMed] [Google Scholar]
- Yoo P, Woock J, Grill W. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Experimental Neurology. 2008;212(1):218–225. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo PB, Horvath EE, Amundsen CL, Webster GD, Grill WM. Multiple pudendal sensory pathways reflexly modulate bladder and urethral activity in patients with spinal cord injury. The Journal of Urology. 2011;185(2):737–743. doi: 10.1016/j.juro.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]


